
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP/EP
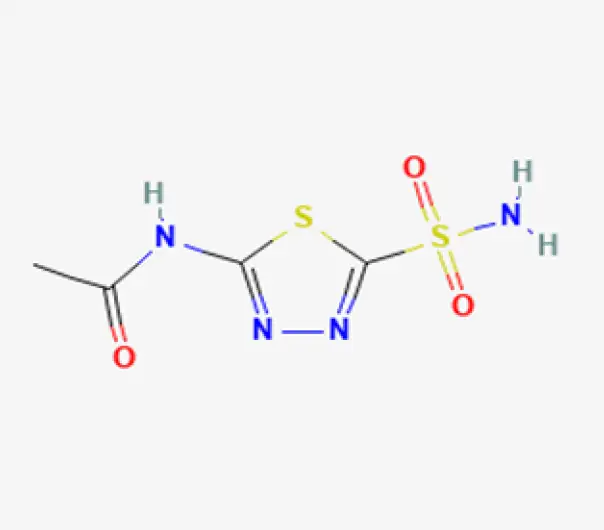
C4H6N4O3S2
N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide
59-66-5
222.25 g/mol
Carbonic anhydrase inhibitors
Diuretics
| Appearance | White to faintly yellow crystalline powder |
|---|---|
| Solubility | Slightly soluble in alcohol; Insoluble in chloroform and ether; Soluble in dilute alkali solutions |
| Melting Point | 255–265 °C (with decomposition) |
| pH (10% solution) | 4.5–6.0 |
Acetazolamide is a carbonic anhydrase inhibitor used across ophthalmology, neurology, nephrology, and altitude medicine. It appears as a white to faintly yellow crystalline powder and is sparingly soluble in water.
Acetazolamide inhibits the enzyme carbonic anhydrase, reducing the formation of hydrogen and bicarbonate ions.
This leads to:
Reduced aqueous humor formation → ↓ intraocular pressure
Increased bicarbonate excretion → diuresis
Mild metabolic acidosis → ↑ ventilation
Neuronal stabilization → antiepileptic effect
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Salius Pharma offers Acetazolamide that complies with BP, USP, and EP pharmacopeial standards, ensuring global acceptability. Each batch undergoes stringent quality checks, impurity profiling, and stability monitoring to ensure consistent, reproducible quality.
We maintain controlled production cycles, validated processes, and GMP-compliant facilities. With strong export capabilities and ready documentation, Salius ensures consistent supply, minimal lead times, and uninterrupted availability for international clients.
Standard packaging includes 25 kg HDPE drums with double-liner protection to maintain product integrity. Customized packaging is available for R&D, pilot batches, or regulatory submissions.
Yes. We provide COA, MSDS, stability data, MOA, TDS, and process-related documents as needed. Our technical team supports clients throughout regulatory submissions and product development stages.
Yes, we offer sample quantities for formulation development, stability studies, and pilot manufacturing. Clients can request samples through our technical or commercial team.
We maintain controlled production cycles, validated processes, and GMP-compliant facilities. With strong export capabilities and ready documentation, Salius ensures consistent supply, minimal lead times, and uninterrupted availability for international clients.
Looking to source Acetazolamide or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.