
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
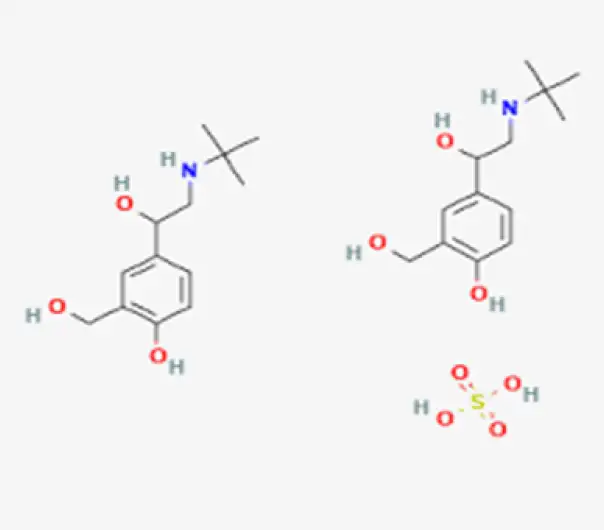
Active Pharmaceutical Ingredient (API)
BP / USP / EP
C₁₃H₂₁NO₃ • H₂SO₄
(RS)-1-(4-hydroxy-3-hydroxymethylphenyl)-2-(tert-butylamino)ethanol sulfate
51022-70-9
576.71 g/mol
Short-Acting β₂-Adrenergic Receptor Agonist (SABA)
Bronchodilator – Asthma & COPD Management
| Appearance | White to almost white crystalline powder / solid |
|---|---|
| Solubility | Soluble in water; slightly soluble in ethanol |
| Melting Point | ~180 °C |
| pH | - |
Albuterol Sulfate, also known as Salbutamol Sulfate, is a high-quality pharmaceutical active ingredient widely used in the formulation of inhalers, nebulizer solutions, tablets, and syrups for the treatment of respiratory disorders. It is a short-acting β₂-agonist (SABA) that delivers fast and effective bronchodilation, making it an essential medicine for asthma and COPD care.
Albuterol Sulfate works by:
Selectively activating β₂-adrenergic receptors in the bronchial smooth muscle
Increasing cAMP levels via adenylate cyclase activation
Leading to rapid bronchodilation and smooth muscle relaxation
Inhibiting release of inflammatory mediators like histamine
Improving airflow and reducing respiratory distress
This fast-acting bronchodilator is widely used as a rescue therapy in acute asthma attacks and exercise-induced bronchospasm.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Salius Pharma specializes in exporting pharma-grade Albuterol Sulfate API supported by GMP-compliant manufacturing and rigorous in-house quality systems. We ensure consistent purity and stability suitable for respiratory pharmaceutical formulations including tablets, inhalers, and syrups.
We provide secure HDPE or fibre drums with double or triple protective liners to prevent humidity exposure during long-distance sea freight. Packaging meets international standards with proper labeling, seals, and palletization to avoid contamination or handling damage.
Yes — Salius Pharma offers COA, MSDS, Stability Data, TSE/BSE declaration, and DMF support as required. We assist buyers by offering technical documentation aligned with LATAM regulatory frameworks to ease product registration and dossier submissions.
Our Albuterol Sulfate has a typical shelf life of 36–60 months, based on proper storage in a cool, dry, and sealed environment. Stability studies confirm suitability even for regions with elevated temperatures and humidity like Latin America.
Every production batch undergoes HPLC purity testing, impurity profiling, related substances, residual solvents analysis, and microbial testing.
Yes — we ship via Air or Sea freight based on urgency and cost preferences. We coordinate with certified freight partners, and support customs documentation including COO, packing lists, and commercial invoices suitable for LATAM import laws.
We bring competitive pricing, strong compliance, consistent availability, and buyer-focused service. With years of export experience, we aim to build long-term partnerships with LATAM clients by ensuring reliability, transparency, and prompt response to every requirement.
Looking to source Albuterol Sulfate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.