
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
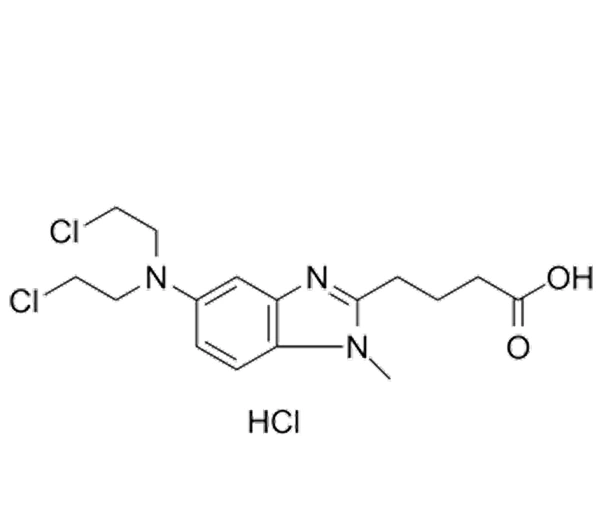
Bendamustine Hydrochloride
C₁₆H₂₁Cl₂N₃O₂ · HCl
458.4 g/mol
3543-75-7
1H-benzimidazole-2-butanoic acid, 4-[5-[bis(2-chloroethyl)amino]-1-methyl-2-benzimidazolyl]-, monohydrochloride
Nitrogen mustard derivative
Anticancer
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Soluble in water; slightly soluble in methanol & ethanol |
| Melting Point | ~150–158°C (decomposes) |
| pH | 3.0 – 4.0 |
Bendamustine Hydrochloride is a nitrogen mustard–based alkylating agent used as an antineoplastic drug. It is commonly utilized in the treatment of various hematological malignancies due to its unique hybrid structure combining alkylating and purine analog properties.
Bendamustine acts primarily as an alkylating agent, forming DNA cross-links that disrupt DNA synthesis and repair. This leads to apoptosis in rapidly dividing malignant cells. Its purine-like structure also contributes to additional antimetabolite activity, making it highly effective in lymphoid cancers.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Bendamustine Hydrochloride is slightly hygroscopic thus the recommended storage is cool, dry place, protected from light and moisture, typically below 25°C. Use moisture-barrier packaging for long-term stability.
Yes, all shipments are accompanied by CoA, MSDS, GMP certificate, and regulatory export documents as required.CTD Modules 2 & 3 or DMF/ASMF support can also be provided for regulated markets.
Bendamustine Hydrochloride is generally compatible with standard pharmaceutical excipients used in solid and injectable formulations. However, due to its alkylating nature, it should not be stored or mixed with reactive excipients (strong oxidizers or nucleophiles) to avoid degradation.
HDPE or fiber drums with double/triple LDPE liners, nitrogen-flushed if required.
Looking to source Bendamustine Hydrochloride or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.