
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
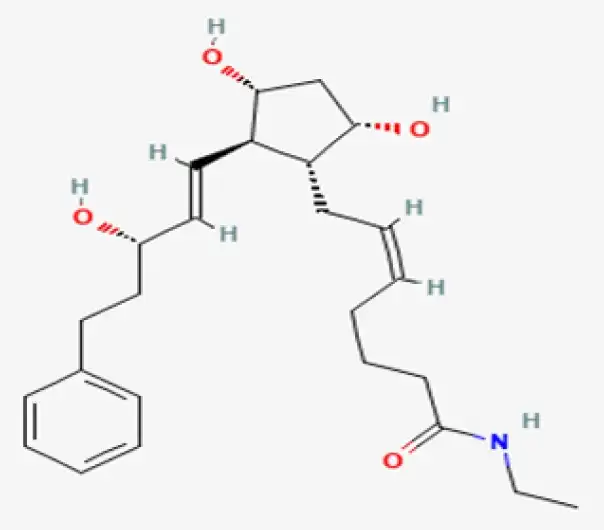
Active Pharmaceutical Ingredient (API)
BP / USP (as applicable)
C25H37NO4
(Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide
155206-00-1
415.57 g/mol
Prostaglandin-related analog
Ophthalmic hypotensive agent
| Appearance | White to off white crystalline / powder solid |
|---|---|
| Solubility | Very soluble in alcohols (e.g. ethanol, methanol), acetone, DMSO; slightly soluble in water |
| Melting Point | 79–80 °C |
| pH | ~5.0–6.0 |
Bimatoprost is a medication used to treat glaucoma by lowering pressure in the eye, and it is also used to promote eyelash growth. It works by increasing the flow of fluid out of the eye, reducing the risk of vision loss.
Bimatoprost is a synthetic prostamide (structurally related to prostaglandin F₂α / prostamides), not acting through classic prostaglandin (FP) receptors. Its exact receptor is not definitively identified. Its ocular hypotensive effect is achieved by increasing outflow of aqueous humour from the eye via both the trabecular meshwork and uveoscleral pathways, thereby lowering intraocular pressure (IOP). When used for eyelash growth (hypotrichosis), bimatoprost is believed to prolong the anagen (growth) phase of hair follicles, thereby increasing length, thickness, and darkness of eyelashes.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
After applying eye drops, Bimatoprost may begin to reduce intraocular pressure within a few hours, with effects lasting up to 24 hours.
Typically as an ophthalmic solution applied carefully to the base of the upper eyelashes once daily (or per doctor’s instructions). It should never be applied directly into the eye for cosmetic use.
Yes — one known side effect is increased pigmentation of the iris or eyelid skin, which may become darker with long-term use.
Bimatoprost is a synthetic prostamide. It is chemically designed in a laboratory and is not extracted from plants or natural sources.
Looking to source Bimatoprost or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.