
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
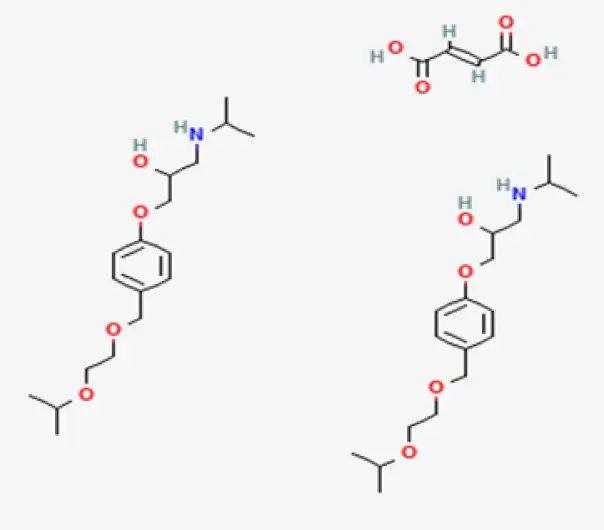
Active Pharmaceutical Ingredient (API)
BP / USP (as applicable)
C18H31NO4
(±)-1-[4-(2-isopropoxyethoxy)phenyl]-3-[(propan-2-yl)amino]propan-2-ol fumarate
66722-44-9
325.43 g/mol (Bisoprolol base), 408.49 g/mol (as fumarate)
Beta-1 selective adrenergic receptor blocker (β1-blocker)
Antihypertensive / Cardiovascular agent
| Appearance | White to off white crystalline solid |
|---|---|
| Solubility | Soluble in water; slightly soluble in alcohol |
| Melting Point | 102–108 °C |
| pH | ~4–5 |
Bisoprolol Fumarate is a selective β1-adrenergic receptor blocker widely used in the management of cardiovascular diseases. It is effective in controlling hypertension, treating angina (chest pain), and supporting heart failure therapy. As a high-quality pharmaceutical API, it is manufactured under strict WHO-GMP, USFDA, and BP/USP standards, making it suitable for global supply to pharmaceutical manufacturers.
Bisoprolol selectively blocks β1-adrenergic receptors in the heart. It reduces heart rate, cardiac output, and myocardial oxygen demand. It lowers blood pressure and helps manage angina and heart failure.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Bisoprolol primarily targets β1-adrenergic receptors, which are mostly located in the heart. This selective action helps reduce heart rate, cardiac output, and blood pressure. However, at higher doses, Bisoprolol may lose some selectivity and affect β2 receptors found in the lungs and blood vessels.
No, Bisoprolol is not addictive, and it does not produce euphoria or dependence. Abrupt discontinuation may lead to rebound high blood pressure, increased heart rate, or worsening chest pain (angina).
Weight gain is not a common side effect, but a small number of patients may experience mild weight changes. This is usually linked to reduced metabolism due to beta-blocker therapy, mild fluid retention, or lower physical activity caused by fatigue (rare).
Better stability during storage and transport, improved solubility helping in uniform tablet formulation, enhanced bioavailability ensuring consistent therapeutic effect, and reduced degradation, especially under humid conditions. In pharmaceutical manufacturing, salts like fumarate are often chosen to achieve optimal processing, longer shelf life, and reliable performance in finished dosage forms.
Yes. Bisoprolol Fumarate requires controlled storage conditions to maintain potency and stability. Keep in a dry, airtight container, protect from light, moisture, and heat, and avoid long exposure to open air due to hygroscopic tendencies.
Looking to source Bisoprolol Fumarate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.