
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
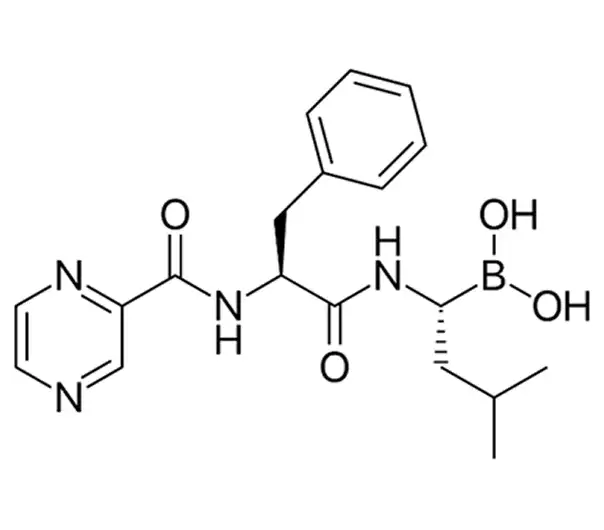
Active Pharmaceutical Ingredient (API)
USP / EP (as applicable)
C19H25BN4O4
(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazin-2-ylcarbonyl)amino]propyl]amino]butylboronic acid
179324-69-7
384.23 g/mol
Boronic acid derivative
Antineoplastic / Anticancer agent
| Appearance | White to off-white powder |
|---|---|
| Solubility | Soluble in DMSO; slightly soluble in methanol; limited solubility in water |
| Melting Point | Approx. 122–126 °C |
| pH | ~4–5 |
Bortezomib is a first-in-class proteasome inhibitor widely used in oncology for the treatment of multiple myeloma and mantle cell lymphoma. It works by blocking the 26S proteasome, leading to controlled cancer cell death. As a boronic acid derivative, Bortezomib has a unique chemical structure that contributes to its potent antineoplastic activity.
Bortezomib reversibly inhibits the 26S proteasome, an enzyme complex responsible for degrading ubiquitinated proteins. This inhibition disrupts protein homeostasis, activates apoptosis (programmed cell death), and selectively targets cancer cells due to their higher protein turnover.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes. Bortezomib is considered a targeted chemotherapy medication. Unlike traditional chemotherapy that attacks rapidly dividing cells in general, Bortezomib specifically blocks the 26S proteasome, a structure cancer cells depend on to manage defective or excess proteins. By disrupting this system, cancer cells accumulate toxic proteins and undergo controlled cell death.
Bortezomib is a fully synthetic pharmaceutical compound. It is created in controlled laboratory and manufacturing environments through multi-step chemical synthesis involving boronic acid derivatives. Because it is synthetic, manufacturers can ensure consistent purity, reproducible quality, controlled impurity levels, and reliable large-scale production.
Bortezomib is given only by trained healthcare professionals. It is commonly administered in two ways: Subcutaneous injection (under the skin), which is widely preferred because it causes fewer side effects, especially neuropathy; and Intravenous (IV) injection, which is used in some treatment protocols but may have a higher risk of infusion-related reactions.
Bortezomib does not cure multiple myeloma, but it is one of the most effective medicines available to control the spread of cancer cells, reduce symptoms, improve organ function, increase overall survival, and maintain long-term disease control.
The API must be stored in airtight containers to limit moisture exposure, light-protective packaging to prevent degradation, and controlled temperatures recommended by pharmacopeias.
Looking to source Bortezomib or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.