
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
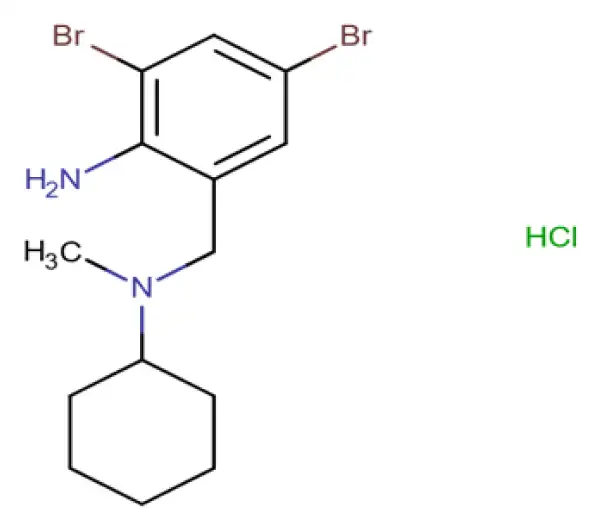
C14H21Br2ClN2
2,4-dibromo-6-{[cyclohexyl(methyl)amino]methyl}aniline hydrochloride
611-75-6
412.59 g/mol
Diphenylmethanes
Laxatives
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; soluble in acetone, ethanol, and chloroform |
| Melting Point | 134–136°C |
| pH | _ |
Bisacodyl is a stimulant laxative used for the temporary relief of occasional constipation and cleansing of the colon as preparation for colonoscopy in adults.

Bisacodyl is deacetylated to the active bis-(p-hydroxyphenyl)-pyridyl-2-methane (BHPM) by an intestinal deacetylase. BHPM can stimulate parasympathetic nerves in the colon directly to increase motility and secretions. Bisacodyl stimulates adenylate cyclase, increasing cyclic AMP, leading to active transport of chloride and bicarbonate out of cells. Sodium ions, potassium ions, and water passively leave the cell, while sodium and chloride ions are unable to be reabsorbed. Bisacodyl directly stimulates parasympathetic nerves in the colon, stimulating contraction of longitudinal smooth muscle but not circular smooth muscle.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Salius Pharma supplies Bisacodyl as a bulk API designed to meet the regulatory requirements and quality standards of Southeast Asian markets. All products are backed by WHO-GMP certification and full regulatory documentation.
Bisacodyl is intended for short-term relief of constipation. Long-term or frequent use without medical supervision may lead to dependency or electrolyte imbalance. It should be used as directed by a healthcare provider.
Bisacodyl stimulates bowel movements by increasing peristalsis in the colon. It typically produces a bowel movement within 6 to 12 hours and is often used before medical procedures like colonoscopies.
Salius Pharma ensures full regulatory support by offering WHO-GMP certification, Drug Master Files (DMFs), Certificate of Analysis (CoA), and region-specific dossiers tailored for approval by authorities in countries like Indonesia, Thailand, Malaysia, Vietnam, and the Philippines.
Common side effects include abdominal cramps, nausea, and diarrhea. Overuse can result in dehydration or electrolyte imbalances. Medical advice is recommended for proper use, especially in vulnerable populations.
Looking to source Bromhexine HCl or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.