
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
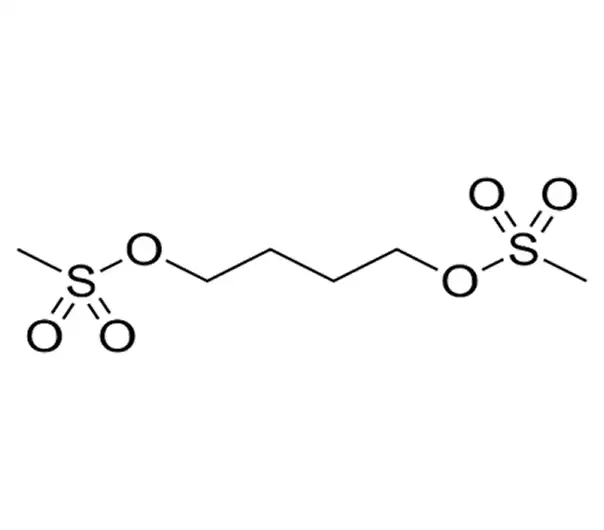
Active Pharmaceutical Ingredient (API)
BP / USP / EP (as applicable)
C6H14O6S2
1,4-Butanediol dimethanesulfonate
55-98-1
246.30 g/mol
Alkylsulfonate / Bifunctional Alkylating Agent
Antineoplastic / Conditioning Agent for Hematopoietic Stem Cell Transplantation
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in chloroform and acetone; slightly soluble in ethanol; practically insoluble in water |
| Melting Point | 220–230 °C |
| pH | - |
Busulfan (Busulphan) is a potent alkylating antineoplastic agent primarily used in oncology. It is one of the most established drugs in conditioning regimens before hematopoietic stem cell transplantation (HSCT), especially for chronic myelogenous leukemia (CML) and other bone marrow disorders. As a bifunctional alkylator, it cross-links DNA, leading to apoptosis of rapidly dividing cells.
Busulfan exerts its effects by forming DNA-DNA crosslinks through alkylation of guanine residues, inhibiting DNA replication and transcription, triggering apoptosis in rapidly dividing hematopoietic cells, and causing long-lasting bone marrow suppression, which is useful in transplant conditioning.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes. Busulfan is a type of chemotherapy known as an alkylating agent, which works by damaging DNA in cancerous and rapidly dividing cells.
Yes. High-Performance Liquid Chromatography (HPLC) is the most widely used method to test Busulfan API purity and potency. Typically, reversed-phase HPLC with UV detection is used to quantify the API and identify any impurities or degradation products.
Busulfan API is sensitive to moisture and light. Recommended standards include high-density polyethylene (HDPE) drums or bottles with airtight closures, aluminum foil or laminated inner lining to prevent moisture ingress, and packaging that complies with WHO-GMP and ICH guidelines for oncology APIs.
Yes. While Busulfan is relatively stable at controlled room temperature, its reactive sulfonate groups make it sensitive to high humidity and temperature fluctuations. For international export, 2–8°C cold chain is often recommended for long-distance shipments or tropical climates. Protection from direct sunlight and heat is mandatory, and temperature-monitoring devices are sometimes used in containers to ensure consistent storage conditions.
Yes, patients are usually advised on some dietary considerations during Busulfan therapy: avoid grapefruit and grapefruit juice, which can interfere with liver metabolism; maintain a well-balanced, protein-rich diet to support recovery from bone marrow suppression; drink adequate fluids to help manage drug excretion and reduce hepatic or renal strain; and limit raw or undercooked foods due to neutropenia, which increases infection risk.
Looking to source Busulfan or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.