
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
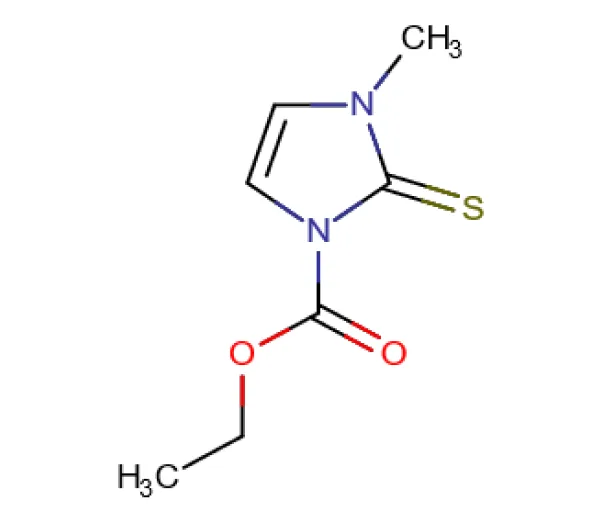
API
BP/USP
C7H10N2O2S
Ethyl 3-methyl-2-sulfanylidene-2,3-dihydro-1H-imidazole-1-carboxylate
22232-54-8
186.23 g/mol
Carbonylimidazoles
Thyroid peroxidase inhibitor
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water, freely soluble in ethanol and chloroform, practically insoluble in ether |
| Melting Point | 173–176 °C |
| pH (1% aqueous solution) | 5.5–6.5 |
Carbimazole is an antithyroid medication used to manage hyperthyroidism and thyrotoxicosis. It is a prodrug that is metabolized into methimazole, the active compound responsible for its therapeutic effect. By inhibiting thyroid hormone synthesis, Carbimazole helps normalize thyroid function and is often used as a preoperative treatment before thyroidectomy or radioiodine therapy.
Carbimazole is converted to methimazole in the body, which inhibits the thyroid peroxidase enzyme. This blocks the iodination of tyrosine residues on thyroglobulin and the coupling of iodotyrosines, reducing the synthesis of the thyroid hormones triiodothyronine (T3) and thyroxine (T4). It also decreases iodine uptake and overall thyroid activity.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Salius Pharma supplies Carbimazole Anhydrous API (≥ 99% purity), BP/USP compliant, to markets across Latin America including Mexico, Argentina, Colombia, and Peru under WHO-GMP standards.
Salius provides DMFs, CoAs, and CTD/eCTD dossiers to support international product registrations and regulatory submissions.
Yes, it is safe when used under medical supervision. Regular monitoring of liver function and blood counts is recommended during prolonged therapy.
Carbimazole is metabolized into methimazole, which inhibits thyroid peroxidase, blocking thyroid hormone synthesis and reducing T3/T4 levels.
Common side effects include rash, nausea, joint pain, and headache. Rare but serious effects include agranulocytosis and liver toxicity, requiring medical monitoring.
Looking to source Carbimazole or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.