
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP / USP / EP
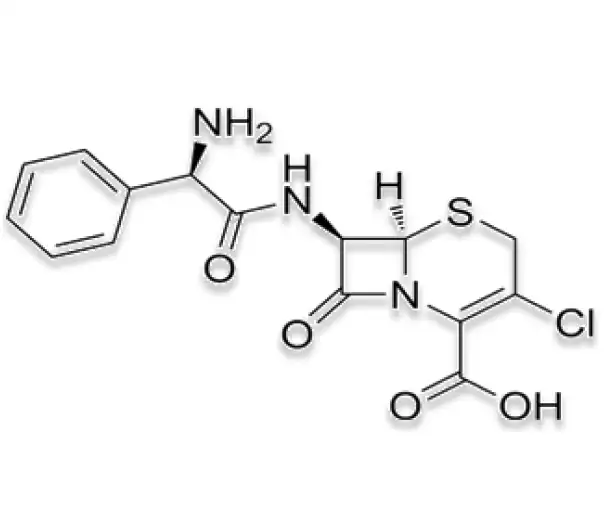
C15H14ClN3O4S
(6R,7R)-7-[(2R)-2-amino-2-phenylacetamido]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
53994-73-3
367.80 g/mol
Second-generation cephalosporin (β-lactam antibiotic)
Antibacterial
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in dilute alkali and certain organic solvents |
| Melting Point | 168–180°C |
| pH (1% aqueous solution) | 3.0–5.0 |
Cefaclor is a second-generation cephalosporin antibiotic that inhibits bacterial cell wall synthesis, exhibiting bactericidal activity against both Gram-positive and selected Gram-negative organisms. It is widely used for respiratory tract, ENT, skin, and urinary infections, and is known for its safety, stability, and broad antimicrobial spectrum. Common formulations include capsules, tablets, suspensions, and pediatric dry syrups.
Cefaclor works by binding to penicillin-binding proteins (PBPs), Inhibiting bacterial cell wall synthesis, Causing cell lysis and death (bactericidal action) , It is stable against several β-lactamases from Gram-positive bacteria but less stable against certain Gram-negative β-lactamases

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes. Cefaclor is a second-generation cephalosporin with activity against both Gram-positive (Streptococcus pneumoniae, S. pyogenes) and selected Gram-negative bacteria (H. influenzae, E. coli, Klebsiella spp.).
Yes. Cefaclor may cause allergic reactions, particularly in patients with penicillin or cephalosporin allergies. Symptoms can include rash, urticaria, or, rarely, anaphylaxis.
Yes. Cefaclor is commonly used for bacterial sinusitis caused by Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis.
The bulk API is stable at room temperature and does not require refrigeration. Reconstituted suspensions should be stored at 2–8°C and used within the recommended duration (typically 7–14 days).
The bulk API typically has a shelf life of 2–3 years when stored properly in a cool, dry place, protected from light and moisture.
Looking to source Cefaclor or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.