
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient + Finished Formulation (Sterile Injection)
USP / BP / EP
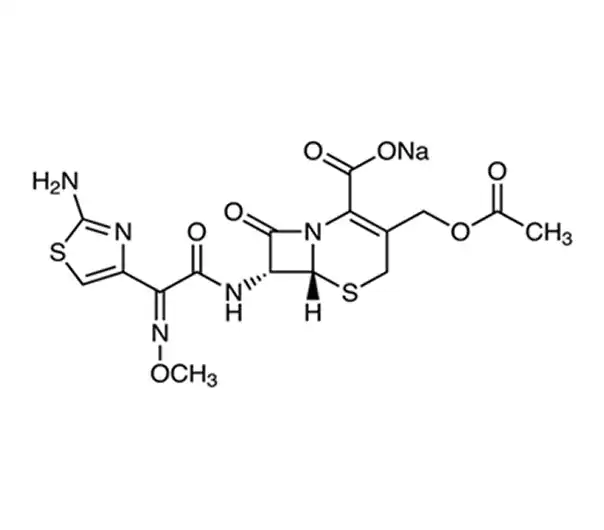
C16H16N5NaO7S2
Sodium (6R,7R)-7-[[(2Z)-2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate-3-yl]methyl acetate
64485-93-4
477.45 g/mol
Third-generation cephalosporin (β-lactam antibiotic)
Broad-spectrum antibacterial for severe Gram-positive & Gram-negative infections
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in dilute alkali and certain organic solvents |
| Melting Point | 168–180°C |
| pH (1% aqueous solution) | 3.0–5.0 |
Cefotaxime Sodium for Injection, USP is a third-generation cephalosporin antibiotic with potent activity against a wide range of Gram-positive and Gram-negative bacteria. It is widely used in hospitals to manage serious infections, including respiratory, septicemic, and central nervous system infections. As a sterile injectable β-lactam antibiotic, Cefotaxime provides fast-acting bactericidal effects and is commonly used for both adult and pediatric patients.
Cefotaxime works by: Binding to Penicillin-Binding Proteins (PBPs) in bacterial cell walls, Inhibiting the final transpeptidation step of peptidoglycan synthesis, Weakening the bacterial cell wall, which leads to cell lysis and rapid bactericidal effect

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, but stability depends on the diluent used. Reconstituted solutions are typically stable for several hours at room temperature and longer if refrigerated.
Cefotaxime Sodium for Injection is packed in USP Type I glass vials, sealed with bromobutyl rubber stoppers and aluminum flip-off seals to ensure sterility and integrity.
Store vials at controlled room temperature, away from light and moisture. Reconstituted solutions should be used promptly or stored as per label guidelines.
Shipments are available under various Incoterms such as FOB, CFR, CIF, or CIP, depending on buyer preference and location.
Yes. Pricing can be shared based on quantity, strength, port of discharge, and packaging requirements.
Looking to source Cefotaxime Sodium for Injection, USP or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.