
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
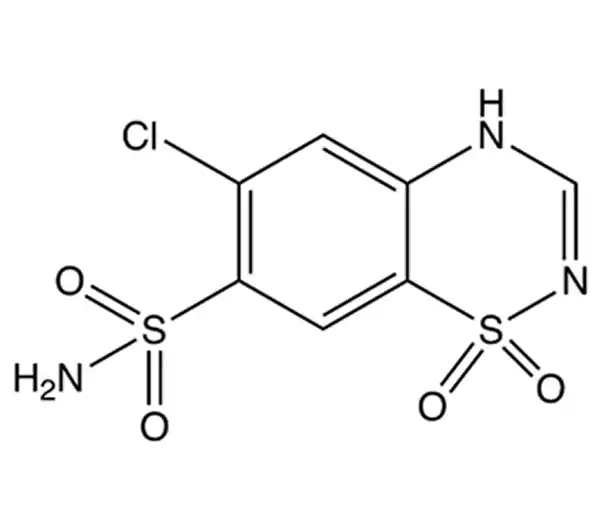
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
C₇H₆ClN₃O₄S₂
295.72 g/mol
58-94-6
6-chloro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
Thiazide diuretic (benzothiadiazine derivative)
Diuretic & antihypertensive agent for edema, hypertension & fluid overload
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in alkaline solutions; sparingly soluble in alcohol; practically insoluble in most organic solvents |
| Melting Point | ~355–360°C |
| pH | ~5.5 – 7.0 |
Chlorothiazide is a first-generation thiazide diuretic widely used in the management of hypertension, edema, congestive heart failure, and renal disorders. It works by blocking sodium reabsorption in the distal convoluted tubule, resulting in increased urine output and reduced plasma volume.
Chlorothiazide acts on the distal convoluted tubule of the nephron by: Inhibiting Na⁺/Cl⁻ symporter → reduces sodium & chloride reabsorption, Increasing urinary excretion of Na⁺, Cl⁻, and water → diuresis. Overall, it provides antihypertensive and anti-edema activity.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes — Open Part DMF / CTD format can be provided for regulated or semi-regulated markets.
250 mg / 500 mg Tablets, 250 mg/5 mL Oral Suspension, 500 mg Injection (lyophilized).
Residual solvents are controlled as per ICH Q3C. Heavy metals & elemental impurities comply with ICH Q3D.
Store at 25°C, protected from excessive moisture & light.
Yes — triple-layer liners, alu-pouches, moisture absorbers, or nitrogen-flushed drums.
Looking to source Chlorothiazide or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.