
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
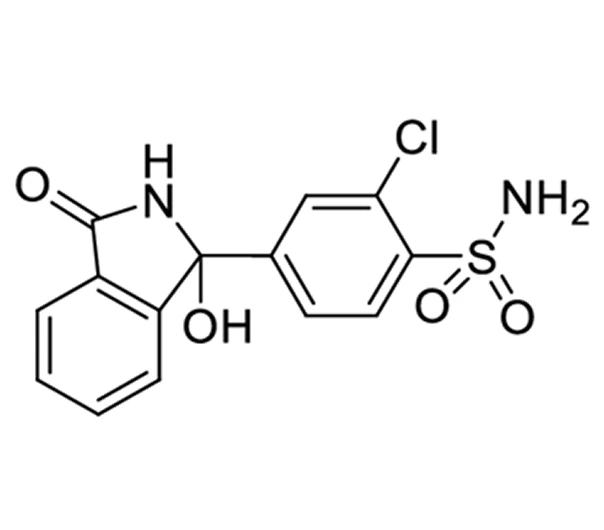
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
C₁₄H₁₁ClN₂O₄S
338.77 g/mol
77-36-1
2-chloro-5-(1-hydroxy-3-oxo-1H-isoindol-2-yl)sulfonylbenzamide
Thiazide-like diuretic (sulfamoylbenzamide class)
Antihypertensive & diuretic for edema and chronic hypertension
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; slightly soluble in methanol; soluble in dilute sodium hydroxide |
| Melting Point | 220–230°C |
| pH | 5.0 – 7.0 |
Chlorthalidone is a thiazide-like diuretic, structurally different from thiazides but providing a longer duration of action, making it one of the most effective drugs for chronic hypertension. It is widely used in monotherapy and fixed-dose combinations (FDCs) with ACE inhibitors, ARBs, beta-blockers, and potassium-sparing diuretics.
Chlorthalidone works primarily in the distal convoluted tubule by: • Inhibiting Na⁺/Cl⁻ symporter, reducing sodium and chloride reabsorption. • Increased urinary excretion of Na⁺, Cl⁻, water → diuresis. • Reduces plasma volume → lower cardiac output → antihypertensive effect.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Hypertension, Prevention of Calcium Kidney Stones, Edema caused by Congestive Heart Failure.
Yes — stable at ambient temperature; avoid excess moisture.
WHO-GMP, COPP, FSC, COO, ISO 9001/14001, Stability data (accelerated + long-term).
Yes — conducted as per ICH Q1B for dossier submissions.
No — Chlorthalidone is typically oral; sterile grade is not commercially used.
IR absorption, HPLC retention time match, UV spectrum identity.
Looking to source Chlorthalidone or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.