
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
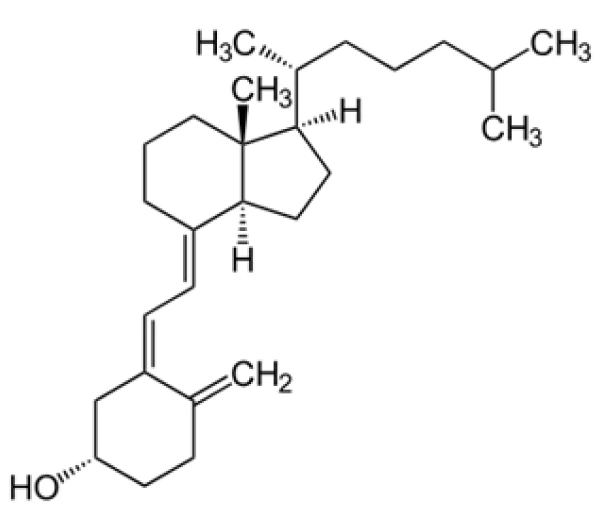
Active Pharmaceutical Ingredient (API)
USP / BP / EP / IP (as applicable)
C₂₇H₄₄O
384.64 g/mol
67-97-0
(3β,5Z,7E)-9,10-secocholesta-5,7,10(19)-trien-3-ol
Secosteroid (Vitamin D analog)
Vitamin supplement for bone health, immunity & calcium metabolism
| Appearance | White to off-white crystalline solid or oily liquid |
|---|---|
| Solubility | Practically insoluble in water; soluble in fats, oils, ethanol & propylene glycol |
| Melting Point | ~82–86°C |
| pH | NA |
Cholecalciferol (Vitamin D₃) is a fat-soluble vitamin essential for calcium and phosphorus absorption, bone formation, immune function, and metabolic balance. It is commonly found in pediatric drops, injectables, fortified meals, nutritional supplements, and high-potency medicinal compositions.
• Absorption: Vitamin D₃ is absorbed in the intestine with dietary fat. • Activation: Converted into active forms in the liver and kidneys: 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D (calcitriol). • Action: Enhances calcium & phosphate absorption from the GI tract, improves bone mineralization, and regulates immune & endocrine functions.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Vitamin D deficiency, Osteoporosis, osteopenia, rickets, osteomalacia, Hypocalcemia, Chronic kidney disease-related bone disorders, Immune support & supplementation in pregnancy.
COA with full analytical data, GMP certificate, ISO, HACCP for premixes, Stability data under ICH conditions.
<0.5% for solid API determined by Karl Fischer titration.
Yes — synthetic from lanolin or vegan/lichen-derived on request.
API can be stored for 24–36 months under proper storage.
Looking to source Cholecalciferol or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.