
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Citalopram Hydrobromide
C20H21FN2O·HBr
405.3 g/mol
59729-32-7
(1RS)-1-(3-Dimethylaminopropyl)-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile hydrobromide
Selective serotonin reuptake inhibitor (SSRI)
Antidepressant
| Appearance | white to off-white crystalline powder |
|---|---|
| Solubility | sparingly soluble in water and soluble in ethanol |
| Melting Point | 182°C to 188°C |
| pH | 4.0 to 6.0 |
Citalopram hydrobromide (HBr), commonly known by the brand name Celexa, is a widely prescribed antidepressant that belongs to a class of drugs called selective serotonin reuptake inhibitors (SSRIs). It works by increasing the levels of serotonin, a natural substance in the brain that helps maintain mental balance, thereby alleviating symptoms of depression.
Citalopram hydrobromide works as a Selective Serotonin Reuptake Inhibitor (SSRI) by blocking the serotonin transporter (SERT), preventing the reabsorption of serotonin (5-HT) back into the neuron, which increases serotonin levels in the synaptic cleft, enhancing neurotransmission and improving mood
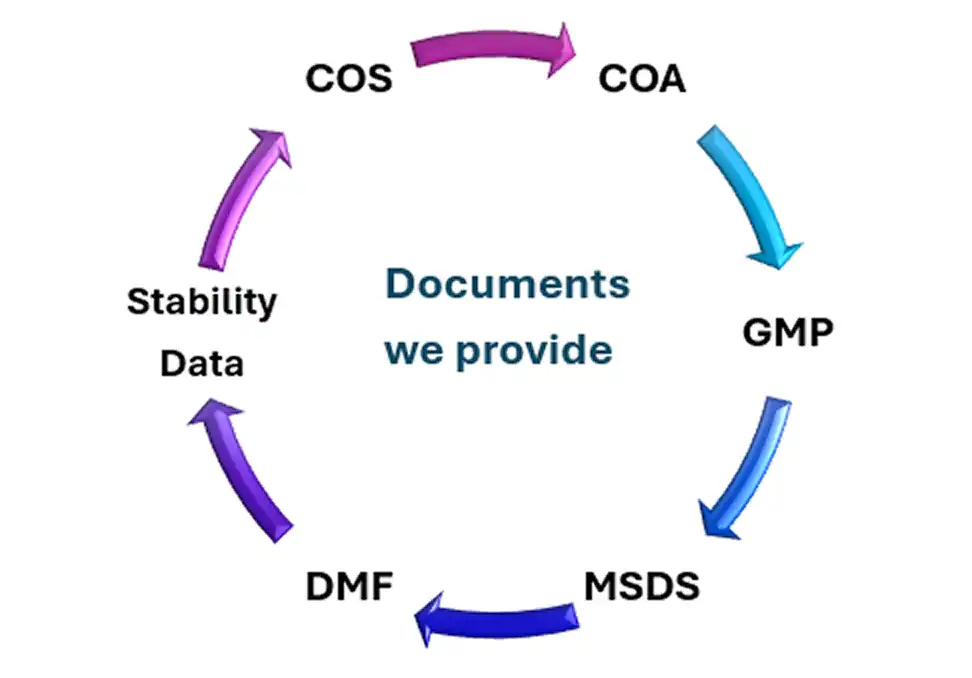
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
The API may exist in specific crystalline forms, which are tightly controlled during manufacturing to ensure consistent solubility, stability, and bioavailability in finished dosage forms.
The API is stable under recommended conditions when protected from moisture and light, with stability data generated under ICH guidelines to support shelf life for global distribution.
Citalopram Hydrobromide API is typically packed in double polyethylene bags within fiber or HDPE drums, ensuring protection against contamination and moisture during international transit.
Looking to source Citalopram HBr or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.