
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
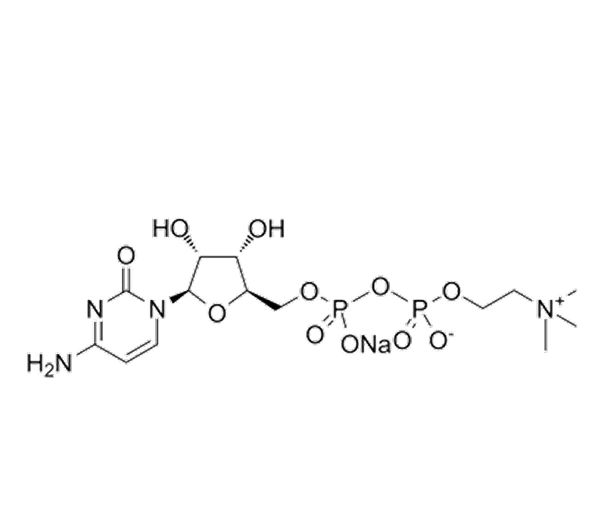
Citicoline Sodium
C₁₄H₂₅N₄NaO₁₁P₂
~510.31 g/mol
33818-15-4
Sodium ({[(2R,3S,4R,5R)-5-(4-aminopyrimidin-2-yl)-4-hydroxy-3-(hydroxymethyl)oxolan-2-yl]methoxy}hydroxyphosphoryl)oxymethanolate
Nucleotide
Neuroprotective
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water, forming clear, colorless solutions, but generally insoluble in ethanol or acetone |
| Melting Point | 259-268°C (decomposes) |
| pH | 6.5-7.5 |
Citicoline sodium (also known as CDP-choline) is an endogenous, naturally occurring compound that serves as a crucial intermediate in the biosynthesis of phosphatidylcholine, a primary component of nerve cell membranes.
Citicoline sodium primarily act as a precursor for phosphatidylcholine (PC), vital for neuronal membrane repair, and as a choline donor for acetylcholine (ACh) synthesis, boosting memory/learning neurotransmission; it also reduces inflammatory damage and oxidative stress, stabilizing cells and improving outcomes in neurological conditions like stroke by supporting membrane integrity and neurotransmitter balance.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Citicoline Sodium increases levels of acetylcholine and other neurotransmitters, supporting cognitive function and neuronal repair.
It is widely used in the management of stroke, traumatic brain injury, cognitive impairment, Parkinson’s disease, and other neurodegenerative or cerebrovascular disorders.
Key parameters include assay, related substances, residual solvents, inorganic impurities, sodium content, water content, and microbial limits, all controlled as per USP/EP/BP specifications.
Citicoline Sodium API can be supplied with batch-wise CoA, MSDS, GMP certificates, stability data, and CTD/DMF documentation to support registrations in regulated and semi-regulated markets such as the US, EU, LATAM, MENA, and Africa.
Looking to source Citicoline Sodium or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.