
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
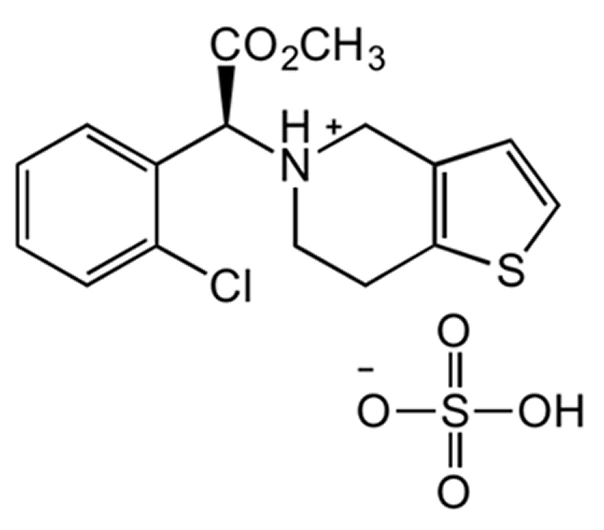
Clopidogrel Bisulfate
C₁₆H₁₆ClNO₂S·H₂SO₄
419.9 g/mol
113665-84-2
(±)-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetic acid methyl ester hydrogen sulfate
Thienopyridine derivative
Antiplatelet
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Practically insoluble in water at neutral pH but becomes freely soluble in acidic conditions |
| Melting Point | 172°C to 184°C |
| pH | 1-3 |
Clopidogrel bisulfate, is an oral antiplatelet agent used to prevent blood clots in patients with cardiovascular diseases. It is a prodrug that becomes active in the liver and works by irreversibly blocking a specific receptor on platelets.
Clopidogrel bisulfate works by its active metabolite irreversibly inhibiting the P2Y12 ADP receptors on platelets. This action prevents platelet aggregation and the formation of blood clots that can lead to heart attacks and strokes.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Clopidogrel is commonly supplied as the bisulfate salt, which offers improved stability, solubility, and bioavailability for oral dosage formulations.
The API should be stored in a cool, dry place protected from light and moisture. Stability data under ICH conditions are available to support shelf life and export requirements.
Yes, the API is typically packed in moisture-proof, double-layered polyethylene or HDPE drums to prevent contamination and ensure quality during transit.
Yes, the API is supplied with comprehensive regulatory documentation including CoA, MSDS, GMP compliance certificates, and DMF/CTD dossiers to facilitate approvals in markets such as the US, EU, LATAM, MENA, and Africa.
Looking to source Clopidogrel Bisulfate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.