
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
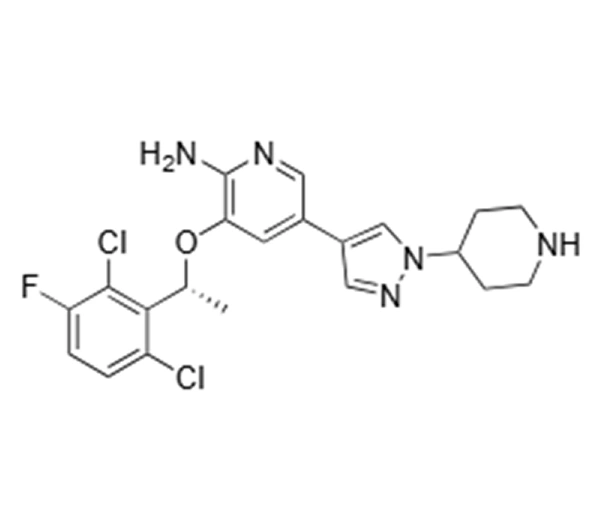
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Crizotinib
C₂₁H₂₂Cl₂FN5O
~450.34 g/mol
877399-52-5
3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethyl]-5-(1-piperidin-4-yl-1H-pyrazol-3-yl)pyridin-2-amine
Kinase inhibitor
Antineoplastic / Targeted cancer therapy
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Practically insoluble in water at neutral pH but becomes freely soluble in acidic conditions |
| Melting Point | 172°C to 184°C |
| pH | 1-3 |
Crizotinib is a targeted cancer drug, specifically a tyrosine kinase inhibitor (TKI), used mainly for ALK-positive non-small cell lung cancer.
Crizotinib’s mechanism involves blocking abnormal signals from ALK, ROS1, and MET proteins, which are often overactive in certain cancers, thereby halting tumor growth and promoting cancer cell death. It works by competitively inhibiting ATP binding in these kinases, stopping cell proliferation and survival signals.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Crizotinib is synthesized through multi-step organic reactions as a small-molecule tyrosine kinase inhibitor, classified under kinase inhibitors targeting ALK, ROS1, and MET pathways.
The API has limited aqueous solubility, so solubility-enhancing techniques like salt formation, micronization, or solid dispersions are often used in oral dosage forms.
High-performance liquid chromatography (HPLC) and chiral analysis are used to quantify assay, related impurities, and confirm enantiomeric purity as per USP/EP/BP standards.
Export requires GMP-compliant manufacturing, detailed stability data, batch-wise CoA, MSDS, and DMF/CTD documentation to facilitate approvals in global markets including US, EU, LATAM, MENA, and Africa.
Looking to source Crizotinib or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.