
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
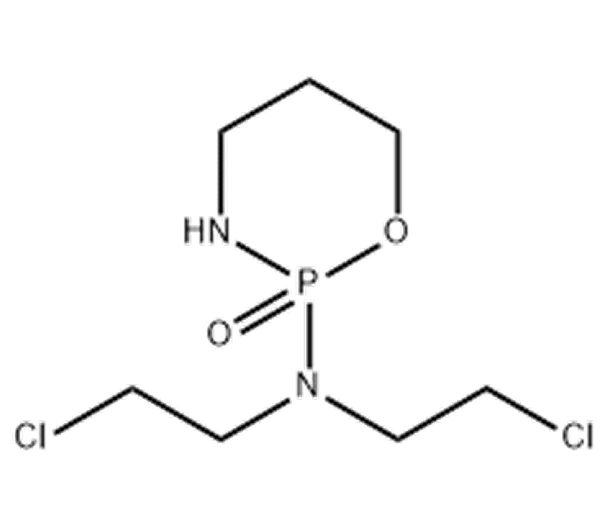
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Cyclophosphamide Anhydrous
C₇H15Cl₂N₂O₂P
~261.09 g/mol
6055-19-2
2-[bis(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine-2-oxide
Nitrogen mustard derivative
Antineoplastic agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Practically insoluble in water at neutral pH but becomes freely soluble in acidic conditions |
| Melting Point | 41-45°C |
| pH | 3.9-7.1 for a 1% solution |
Cyclophosphamide is a potent alkylating agent used in chemotherapy and as an immunosuppressant. It is administered as an inactive prodrug that requires metabolic activation in the liver to form its active metabolites, which work by damaging the DNA of rapidly dividing cells.
Cyclophosphamide is metabolized by cytochrome P450 (CYP) enzymes, mainly CYP2B6, in the liver. This process converts the inactive parent drug into active intermediate metabolites, primarily 4-hydroxycyclophosphamide, which exists in equilibrium with its tautomer, aldophosphamide.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Cyclophosphamide Anhydrous is a prodrug that is metabolized in the liver to active alkylating metabolites. These metabolites form covalent bonds with DNA, causing cross-linking, inhibiting DNA replication and transcription, and ultimately triggering apoptosis in rapidly dividing cancer cells.
The anhydrous form contains less moisture, offering better stability and longer shelf life, which is advantageous for international shipment and formulation without compromising therapeutic efficacy.
For export, the API is supplied with batch-specific Certificates of Analysis (CoA), MSDS, GMP compliance certificates, stability data, and DMF/CTD dossiers to facilitate regulatory approvals in global markets.
The API can support registrations and supply regulated and semi-regulated markets, including the US, EU, LATAM, MENA, and Africa, with documentation meeting local pharmacopeial and regulatory requirements.
Looking to source Cyclophosphamide Anhydrous or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.