
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
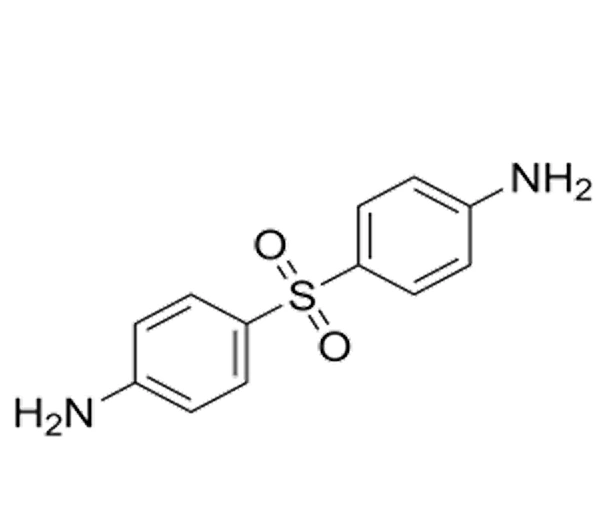
Active Pharmaceutical Ingredient (API)
C₁₂H₁₂N₂O₂S
248.33 g/mol
80-08-0
4,4’-Diaminodiphenyl sulfone
Sulfone derivative
Antibacterial, Anti-leprotic, Dermatologic agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in acetone, alcohol |
| Melting Point | 172–174°C |
| pH | 6–7 |
Dapsone is a synthetic sulfone antibiotic with anti-inflammatory and antibacterial properties. It is primarily used in the treatment of leprosy (Hansen’s disease), dermatitis herpetiformis, and certain pneumocystis infections.It acts on bacterial folate synthesis and exhibits immunomodulatory effects, making it useful in dermatological inflammatory conditions.
Dapsone inhibits dihydropteroate synthase, an enzyme in the bacterial folate synthesis pathway. It themn prevents the formation of dihydropteroic acid, which is necessary for nucleic acid synthesis. It is bacteriostatic against Mycobacterium leprae and other susceptible bacteria.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Primarily bacteriostatic, especially against Mycobacterium leprae; higher concentrations may have bactericidal effects.
Yes, effective against some gram-positive bacteria, Pneumocystis jirovecii, and certain protozoa.
It is part of multidrug therapy (MDT), usually combined with rifampicin and clofazimine.
Standard pharmaceutical handling is sufficient; avoid inhalation of dust and use PPE for bulk handling.
Looking to source Dapsone or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.