
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
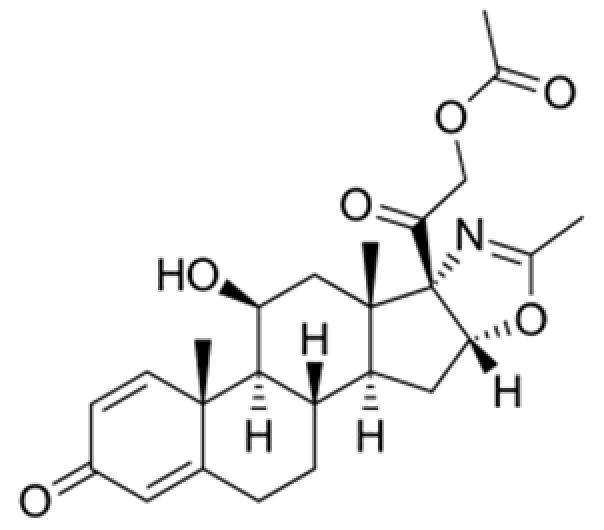
Active Pharmaceutical Ingredient (API)
C₂₆H₃₅FO₆
392.56 g/mol
66733-05-5
(11β,16α)-9-Fluoro-11,21-dihydroxy-16-methylpregna-1,4-diene-3,20-dione
Glucocorticoid, corticosteroid derivative
Anti-inflammatory, immunosuppressive agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in ethanol, methanol, DMSO |
| Melting Point | 250–254°C |
| pH | 6–7 |
Deflazacort is a synthetic glucocorticoid with potent anti-inflammatory and immunosuppressive properties. It is used in the treatment of:
Autoimmune diseases (e.g., rheumatoid arthritis, lupus)
Duchenne Muscular Dystrophy (DMD) – to slow disease progression
Deflazacort is considered less metabolically disruptive than prednisone, causing fewer side effects like weight gain and hyperglycemia.
Deflazacort binds to glucocorticoid receptors (GRs) in the cytoplasm → forms a steroid-receptor complex → translocates to the nucleus. It regulates gene transcription, upregulating anti-inflammatory proteins and downregulating pro-inflammatory cytokines. It also, inhibits phospholipase A₂, reducing arachidonic acid release → decreases prostaglandins and leukotrienes.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, it reduces lymphocyte proliferation, cytokine release, and inflammatory mediator production.
Deflazacort is metabolized hepatically via hydrolysis to its active metabolite.
Yes, stability data under ICH Zone III/IV conditions is available.
Yes, we provide complete technical and regulatory dossier support.
Yes, it is a controlled glucocorticoid requiring prescription in most countries.
Looking to source Deflazacort or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.