
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
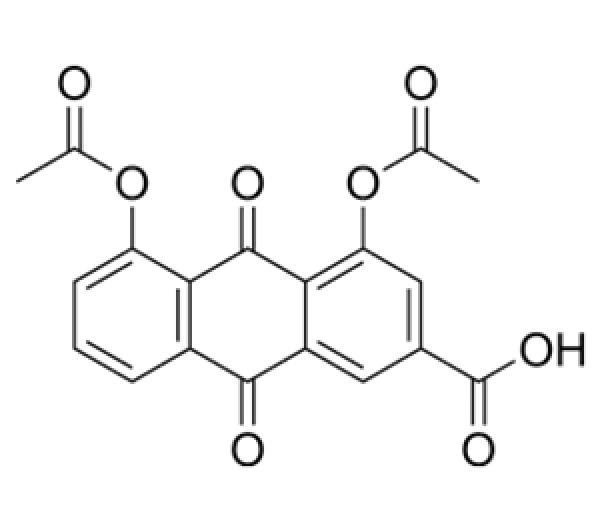
Active Pharmaceutical Ingredient (API)
C₁₉H₁₀O₇
368.28 g/mol
138-54-7
4,5-diacetyloxy-9,10-dioxo-anthracene-2-carboxylic acid
Anthraquinone derivative
Symptomatic slow-acting drug for osteoarthritis (SYSADOA); chondroprotective agent
| Appearance | Yellow to orange crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in ethanol, acetone |
| Melting Point | 252–256°C |
| pH | 4–5 |
Diacerein is a slow-acting symptomatic drug for osteoarthritis (SYSADOA) with anti-inflammatory and chondroprotective properties. It is primarily used to:
• Reduce joint pain and stiffness in osteoarthritis
• Protect cartilage from degradation
It is considered an alternative to NSAIDs for long-term management, especially when avoiding gastrointestinal side effects.
Primary Action: Inhibits interleukin-1β (IL-1β) activity, a major mediator in cartilage degradation.
Reduces matrix metalloproteinases (MMPs), which break down cartilage extracellular matrix.
Exhibits anti-inflammatory effects by reducing pro-inflammatory cytokines in synovial fluid.
Stimulates synthesis of proteoglycans and collagen in cartilage.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It inhibits interleukin-1β (IL-1β) activity, a key cytokine responsible for cartilage degradation and synovial inflammation.
No significant effect; considered safe in metabolic comorbidities.
You can always start with low-dose therapy, take with meals, and maintain adequate hydration.
2–4 weeks depending on order size and documentation.
DMF/CTD dossier, GMP certificate, CoA (3 batches), MSDS, and stability data.
Looking to source Diacerein or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.