
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
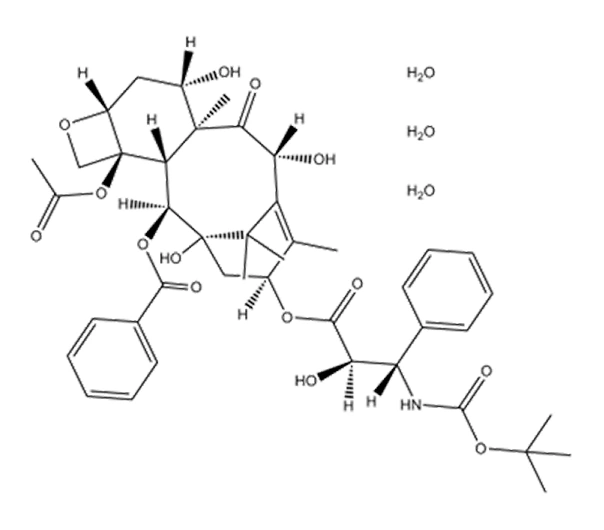
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Docetaxel Trihydrate
C₄₃H₅₃NO₁₄·3H₂O
861.9 g/mol
114977-28-5
(2R,3S)-N-Carbobenzyloxy-3-phenylisoserine, 13-ester with 10-deacetylbaccatin III 2-benzoate, trihydrate
Taxane derivative
Antineoplastic agent
| Appearance | white to off-white crystalline powder |
|---|---|
| Solubility | practically insoluble in water at neutral pH but becomes freely soluble in acidic conditions |
| Melting Point | > 170 °C |
| pH | - |
Docetaxel trihydrate is a potent anticancer chemotherapy drug that belongs to the taxane family and is derived semi-synthetically from the European yew tree. It is used to treat various solid tumors, including breast, non-small cell lungs, prostate, gastric, and head and neck cancers.
Docetaxel trihydrate is an antineoplastic (anticancer) agent that primarily works as a microtubule inhibitor. Its main mechanism of action involves disrupting the normal function of the cellular microtubule network, which is essential for cell division.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Docetaxel Trihydrate is manufactured in cGMP-compliant facilities with validated processes and strict control of critical process parameters, impurities, residual solvents, and microbial limits in accordance with ICH guidelines. Each batch is released with a Certificate of Analysis to ensure consistent quality for international markets.
It is a white to off-white crystalline powder, sensitive to light, heat, and moisture, supplied in a stable trihydrate form.
Manufactured under cGMP, meeting ICH limits for impurities, residual solvents, and heavy metals, with batch CoA and stability data.
Stored at 2–8°C, protected from light, packed in double PE bags inside HDPE/fiber drums with cold-chain shipping.
Supplied with DMF, CTD Modules 2 & 3, GMP certificate, and full export documents for US, EU, LATAM, MENA, and Africa.
Looking to source Docetaxel Trihydrate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.