
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
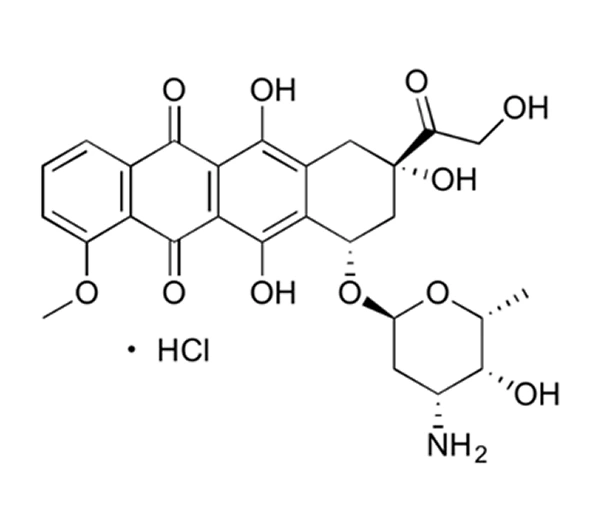
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Doxorubicin Hydrochloride
C₂₇H₂₉NO₁₁·HCl
579.99 g/mol
25316-40-9
(8S,10S)-10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione hydrochloride
Anthracycline glycoside
Antineoplastic agent
| Appearance | Red to dark red crystalline powder |
|---|---|
| Solubility | Freely soluble in water, soluble in methanol, slightly soluble in ethanol |
| Melting Point | 195–197°C |
| pH | 2.5–4.5 |
Doxorubicin hydrochloride is an anthracycline chemotherapy drug that treats various cancers by damaging cancer cell DNA and inhibiting key enzymes needed for cell growth. It is used in the treatment of a wide range of cancers, including breast cancer and leukemias.
Doxorubicin's mechanism involves multiple actions: it intercalates (inserts) into DNA, blocking replication and transcription; it inhibits topoisomerase II, preventing DNA repair and causing breaks; and it generates free radicals that damage DNA and cell membranes, ultimately leading to cancer cell death (apoptosis).
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Each shipment includes Certificate of Analysis (CoA), Material Safety Data Sheet (MSDS), Certificate of Origin (COO), and packing list, ensuring compliance with international trade requirements.
Yes, the API is manufactured to meet USP, BP, and EP standards, making it suitable for regulated and semi-regulated markets worldwide.
Doxorubicin Hydrochloride should be stored at 2–8°C, protected from light and moisture, to maintain stability and potency during storage.
For export, the API is packed in double polyethylene bags inside HDPE or fiber drums and shipped using validated cold-chain logistics to ensure it remains stable and compliant during transit.
Looking to source Doxorubicin Hydrochloride or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.