
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient (API)
BP / USP / EP (as applicable)
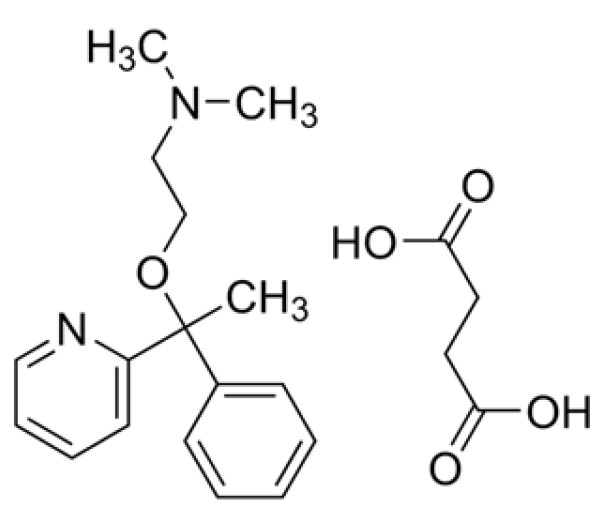
Doxylamine Succinate
C₁₇H₂₂N₂O·C₄H₆O₄
~388.46 g/mol
562-10-7
N,N-dimethyl-2-(1-phenyl-1-pyridin-2-ylethoxy)ethanamine hydrogen succinate
First-generation ethanolamine antihistamine
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water & alcohol; slightly soluble in chloroform |
| Melting Point | 107–111°C |
| pH | 5.0 – 6.0 |
Doxylamine Succinate is a first-generation antihistamine from the ethanolamine class with pronounced sedative properties. It is widely used globally in OTC formulations for allergic symptoms, insomnia, and as a night-time analgesic combination component.
It acts in the following 3 ways:
1. H₁-receptor antagonist: Blocks histamine H₁ receptors → reduces allergic symptoms.
2. Anticholinergic action: Mild M₁ receptor blockade contributes to sedative and antiemetic effects.
3. CNS depressant: Strong central histamine blockade → sleep-inducing properties.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, Doxylamine Succinate is compatible with common excipients such as lactose, MCC, and starch. Mild hygroscopicity requires avoiding very high-moisture excipients.
Generally, wet granulation is preferred for uniformity and better flow. Direct compression is possible with optimized PSD and flow aids.
It is stable between pH 4.5–6.0. Liquid formulations typically target pH 5.0–5.5.
Not required as the API isn’t oxygen-sensitive. However, nitrogen flushing can be provided if requested.
No, it is shipped as non-hazardous, non-dangerous goods under IATA/IMDG/ADR.
Looking to source Doxylamine Succinate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.