
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient (API)
USP / EP / In-house
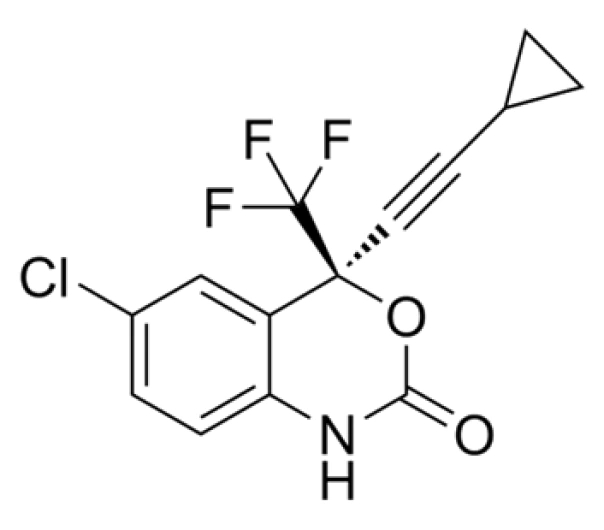
Efavirenz
C₁₄H₉ClF₃NO₂
315.68 g/mol
154598-52-4
(S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl)-2H-3,1-benzoxazin-2-one
Benzoxazinone derivative
Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI)
| Appearance | White to slightly brownish crystalline powder |
|---|---|
| Solubility | Very slightly soluble in water; soluble in DMSO, ethanol, methanol |
| Melting Point | 137–140°C |
| pH | - |
Efavirenz is a first-line NNRTI used in combination antiretroviral therapy (ART) for HIV-1 infection. It is widely used globally due to once-daily dosing and high efficacy in viral load suppression.
Efavirenz works by non-competitively inhibiting HIV-1 reverse transcriptase (RT). It binds to a specific allosteric site on the enzyme, which stops the virus from making DNA. This way, HIV-1 cannot replicate, but it doesn’t affect human DNA polymerases, so our own cells are mostly safe.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, micronized and PSD-controlled grades are available for FDC tablets and formulation consistency. This ensures uniform flow, compressibility, and content uniformity.
Efavirenz is stable under normal light exposure, but opaque drums are recommended for storage and shipping.
Yes, compatible with standard excipients used in tablets and capsules. No significant interaction observed under recommended storage conditions.
Yes, we provide DMF/CEP support, CoA, MSDS, stability reports, and regulatory dossiers tailored for LATAM, MENA, and African countries.
Yes, we can provide HDPE drums, triple poly liners, and optional nitrogen-flushed packing for moisture-sensitive environments.
Looking to source Efavirenz or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.