
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
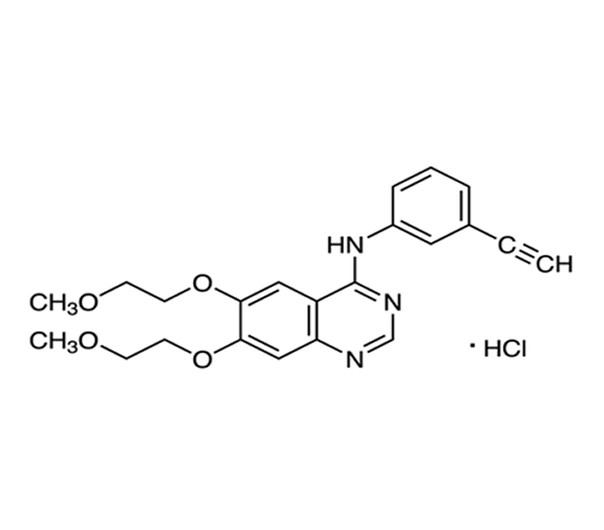
Erlotinib Hydrochloride
C₂₂H₂₃N₃O₄·HCl
429.9 g/mol
183321-74-6
N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine hydrochloride
Quinazoline derivative
Antineoplastic agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water, freely soluble in DMSO, ethanol, and methanol |
| Melting Point | 223–225°C |
| pH | 5.5–7.0 |
Erlotinib hydrochloride is an oral, small-molecule tyrosine kinase inhibitor (TKI) used as a targeted therapy for certain types of cancer, most notably non-small cell lung cancer (NSCLC) and pancreatic cancer. It is not a traditional chemotherapy drug.
Erlotinib Hydrochloride (Tarceva) is a targeted therapy that works as a reversible Epidermal Growth Factor Receptor (EGFR) tyrosine kinase inhibitor (TKI), blocking signals that tell cancer cells to grow and divide by binding to the ATP site of the EGFR, preventing its activation and downstream signaling, leading to cell death, especially in cancers (like NSCLC) with EGFR mutations.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Erlotinib Hydrochloride is manufactured in compliance with USP, BP, and EP pharmacopeial standards, ensuring consistent quality and purity. Its cGMP-compliant production and validated analytical testing make it suitable for regulated and semi-regulated markets worldwide.
The API is produced under strict cGMP conditions, with control over residual solvents, impurities, heavy metals, and microbial limits. Each batch undergoes in-process testing and final release testing, with a batch-specific Certificate of Analysis (CoA) to ensure quality for global supply.
Export-ready documentation includes DMF/ASMF (if applicable), CTD Modules 2 & 3, GMP certificate, CoA, MSDS, Certificate of Origin (COO), and packing list, supporting submissions to US, EU, LATAM, MENA, and African markets.
The API should be stored at 2–8°C, in a dry, light-protected environment, to maintain stability and prevent degradation during storage and transit.
Erlotinib Hydrochloride is packed in double polyethylene bags inside HDPE or fiber drums and shipped using validated cold-chain logistics, ensuring the product remains stable and compliant throughout transportation.
Looking to source Erlotinib Hydrochloride or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.