
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP/NF
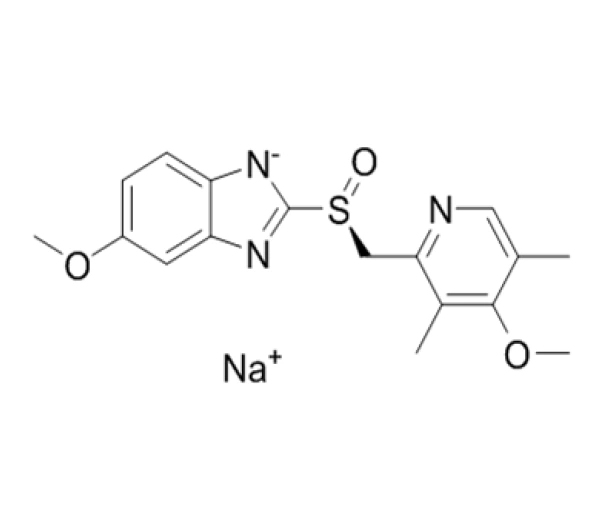
C17H18N3NaO3S
Sodium (S)-5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylsulfinyl]-1H-benzimidazole
161796-78-7
367.39 g/mol
Benzimidazole derivatives
Proton Pump Inhibitor (PPI)
| Appearance | Off-white crystalline solid |
|---|---|
| Solubility | Freely soluble in water; soluble in methanol and DMF |
| Melting Point | 155 – 160°C |
| pH (1% aqueous solution) | 9.0 – 10.0 |
Esomeprazole Sodium is the sodium salt form of Esomeprazole, the S-enantiomer of Omeprazole. It acts as a selective and irreversible proton pump inhibitor (PPI) by inhibiting the H⁺/K⁺-ATPase enzyme in gastric parietal cells, thereby reducing gastric acid secretion. It is widely used for managing gastroesophageal reflux disease (GERD), peptic ulcers, and Zollinger–Ellison syndrome, and for preventing gastric ulcers induced by NSAIDs.
Esomeprazole Sodium selectively inhibits the gastric H⁺/K⁺-ATPase enzyme, which catalyzes the final step of acid production in the stomach. By irreversibly binding to this proton pump, it suppresses both basal and stimulated acid secretion. Being the S-enantiomer of Omeprazole, it provides superior bioavailability, prolonged acid suppression, and more consistent therapeutic outcomes compared to racemic Omeprazole.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is used to treat acid-related conditions including GERD, erosive esophagitis, ulcers, and Zollinger–Ellison syndrome.
Esomeprazole is the S-enantiomer of Omeprazole, offering improved bioavailability and more consistent acid suppression.
Yes. It may interact with clopidogrel, warfarin, and certain antifungal or antiviral drugs. Always consult your physician before use.
Yes. Salius Pharma Pvt. Ltd. exports Esomeprazole Sodium API to multiple Middle Eastern countries including the UAE, Saudi Arabia, and Oman, backed by WHO-GMP certification and regulatory compliance.
Looking to source Esomeprazole Sodium or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.