
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
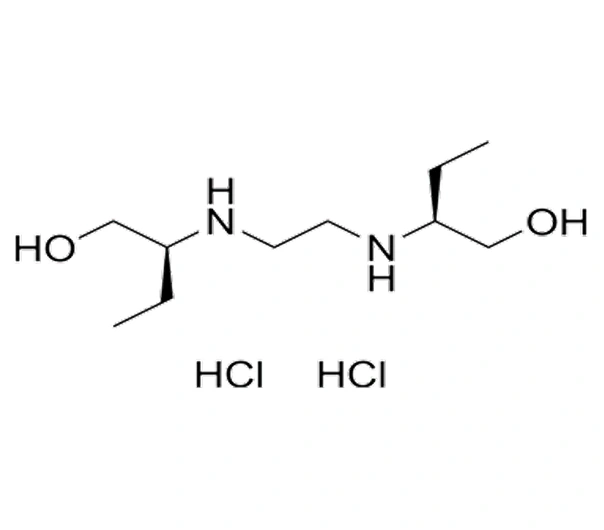
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Ethambutol HCl
C₁₀H₂₄N₂O₂
~204.31 g/mol
74-55-5
(2R,2′R)-2,2′-(ethane-1,2-diyldiimino)di-1-butanol
Amino alcohol
Antitubercular
| Appearance | White crystalline powder |
|---|---|
| Solubility | Freely soluble in water; slightly soluble in alcohol; insoluble in chloroform |
| Melting Point | 208–210°C |
| pH | - |
Ethambutol Hydrochloride is a first-line anti-TB drug used in combination therapy for the treatment of pulmonary and extrapulmonary tuberculosis. It is primarily used in fixed-dose combination (FDC) formulations with Rifampicin, Isoniazid, and Pyrazinamide.
Ethambutol works by inhibiting arabinosyl transferase, an enzyme that is essential for building the mycobacterial cell wall. By stopping the incorporation of arabinose into arabinogalactan, it disrupts the cell wall synthesis, which weakens the bacteria. As a result, Ethambutol is bacteriostatic and mainly prevents the growth of actively dividing mycobacteria.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Supplied as a crystalline form, which ensures stability, consistent solubility, and uniform flow properties for tablet formulation.
Yes, the API is fully compliant with USP, EP, and BP monographs, meeting assay, impurity, and physical specifications for global markets.
Manufactured under cGMP conditions with controlled environment, validated cleaning, and routine microbiological testing to ensure low bioburden.
Yes, each batch is supplied with a complete CoA, MSDS, and stability summary, ensuring traceability and regulatory compliance.
Yes, CTD Modules 2 & 3 can be supplied to support registration and regulatory submissions in LATAM, MENA, and African countries.
Looking to source Ethambutol HCl or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.