
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
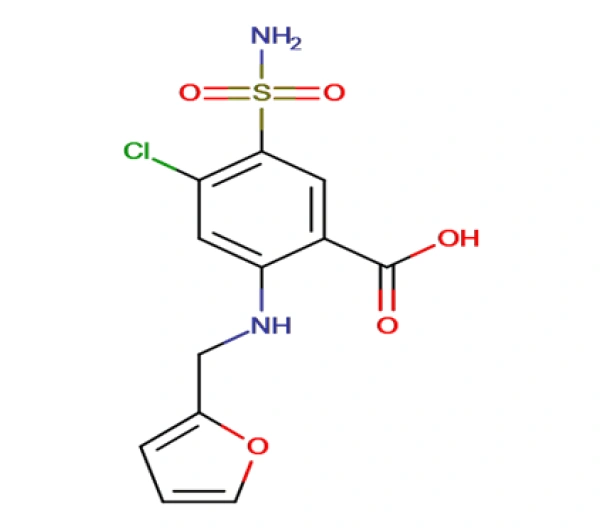
C12H11CLN2O5S
4-chloro-2-{[(furan-2-yl)methyl]amino}-5-sulfamoylbenzoic acid
54-31-9
330.74 g/mol
Sulfonamide derivatives
Diuretic
| Appearance | White to slightly yellow crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; freely soluble in alkaline solutions and methanol |
| Melting Point | 223–226°C |
| pH | 4.0 to 6.0 |
Furosemide is a loop diuretic used to treat edema associated with heart failure, liver disease, and kidney disorders, as well as hypertension. It works by inhibiting the sodium-potassium-chloride (Na⁺/K⁺/2Cl⁻) co-transporter in the thick ascending limb of the loop of Henle in the kidneys, promoting increased urine output and reducing fluid buildup. Furosemide is widely used due to its rapid onset and potent diuretic effect.
Furosemide blocks the Na⁺/K⁺/2Cl⁻ co-transporter in the kidney’s loop of Henle, preventing reabsorption of sodium, potassium, and chloride ions. This leads to increased urine production, reducing excess fluid and lowering blood pressure.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It treats edema, hypertension, and fluid overload conditions.
Rarely, high doses or rapid IV administration may cause hearing loss or tinnitus.
Yes, it can be taken with or without food.
Yes, Salius Pharma Pvt. Ltd. exports Furosemide API to countries across Africa and the Middle East as part of its global supply strategy. The company is an ISO 9001:2015 certified and Government of India-recognized Export House, offering high-quality APIs with regulatory support such as COAs, stability data, and DMFs to meet international market requirements. Their presence in key regions like Nigeria, Kenya, Saudi Arabia, and the UAE reflects their commitment to expanding access to essential APIs like Furosemide.
Yes, they provide technical support, documentation, and consultation for API use and regulatory compliance.
Looking to source Furosemide or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.