
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
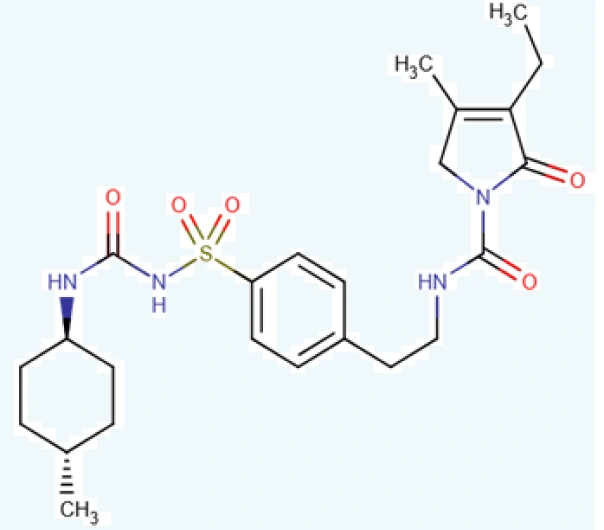
C₂₃H₂₈ClN₃O₅S
3-ethyl-4-methyl-2-oxo-N-(2-{4-[({[(1r,4r)-4- methylcyclohexyl]carbamoyl} amino)sulfonyl]phenyl}ethyl)-2,5-dihydro-1H-pyrrole-1-carboxamide
93479-97-1
490.62 g/mol
Sulfonylurea
Anti Diabetic
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; soluble in dimethyl sulfoxide (DMSO) |
| Melting Point | 170–179°C |
| pH | - |
Glibenclamide also known as Glyburide in the U.S., is a second-generation sulfonylurea used for the oral treatment of type 2 diabetes mellitus. It lowers blood glucose levels by stimulating the release of insulin from pancreatic β-cells. This is achieved through binding to ATP-sensitive potassium channels on the β-cell membrane, leading to depolarization, calcium influx, and insulin secretion. Glibenclamide is more potent than first-generation sulfonylureas and is often used when lifestyle modifications and other oral agents like metformin are insufficient. Due to its longer duration of action, it is typically taken once or twice daily.
Glibenclamide works by stimulating the release of insulin from pancreatic β-cells to lower blood glucose levels in people with type 2 diabetes. It binds to sulfonylurea receptors (SUR1) on ATP-sensitive potassium channels in the β-cell membrane, causing the channels to close. This leads to membrane depolarization and the opening of voltage-gated calcium channels, allowing calcium to enter the cells. The increased intracellular calcium triggers the exocytosis of insulin-containing granules, thereby increasing insulin secretion and reducing blood sugar levels.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Glibenclamide is used to treat type 2 diabetes by stimulating the pancreas to release more insulin and lower blood sugar levels.
Glimepiride should be taken once daily with breakfast or the first main meal of the day, swallowed whole with water, and at the same time each day to maintain stable blood sugar levels. It's important to follow your doctor's prescribed dose exactly and avoid skipping or doubling doses. Eating regularly helps prevent hypoglycemia, especially if you're taking other diabetes medications.
Yes, Glibenclamide can cause low blood sugar (hypoglycemia), which is one of its most common and serious side effects. This happens because it stimulates the pancreas to release insulin regardless of your current blood sugar level. The risk of hypoglycemia increases if you skip meals, exercise excessively, drink alcohol, or take it along with other blood sugar-lowering medications. Symptoms of low blood sugar include sweating, shakiness, dizziness, hunger, confusion, and in severe cases, fainting or seizures. Always take Glibenclamide with food and follow your doctor’s dosing instructions to minimize this risk.
Yes, Salius Pharma Pvt. Ltd. does export Glibenclamide API, and its strategic focus on growing its API business in Africa and the Middle East underscores this availability. They explicitly list Glimepiride among their chronic therapy APIs, and their global expansion strategy includes major markets such as Nigeria, Ghana, Kenya, South Africa, and countries across the Middle East.
Yes, Salius Pharma offers full technical support and regulatory documentation for Glibenclamide API, including DMF, CTD, and CEP formats tailored to international standards. The company supports clients with regulatory submissions such as ANDA, ACTD, and country-specific dossiers, ensuring compliance with WHO-GMP, USFDA, and other global authorities. This enables seamless registration and supply of Glibenclamide API in African and Middle Eastern markets, aligning with Salius Pharma’s strategic export focus in these regions.
Looking to source Glibenclamide or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.