
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Gliclazide
C₁₅H₂₁N₃O₃S
~323.42 g/mol
21187-98-4
1-(Hexahydrocyclopenta[c]pyrrol-2(1H)-ylcarbonyl)-3-ethylsulfonylurea
Sulfonylurea derivative
Oral antidiabetic
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in DMSO and alcohol |
| Melting Point | 182–186°C |
| pH | 3–7 |
Gliclazide is an oral antidiabetic agent from the sulfonylurea class. It is primarily used in the management of type 2 diabetes mellitus to improve glycemic control, either as monotherapy or in combination with other antidiabetic drugs. It acts by stimulating insulin secretion from pancreatic β-cells and may reduce the risk of microvascular complications associated with diabetes.
Gliclazide works by binding to sulfonylurea receptors (SUR1) on pancreatic β-cells, which closes the ATP-sensitive potassium channels. This causes the cell membrane to depolarize, opening calcium channels and increasing the level of calcium inside the cells. The higher intracellular calcium then triggers the release of insulin, which helps lower blood glucose levels in patients with type 2 diabetes.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Direct compression or wet granulation can be used, and micronized grades are recommended for tablet formulations.
Gliclazide is primarily used to manage type 2 diabetes mellitus, either as monotherapy or in combination with other antidiabetic agents, to improve glycemic control.
It is slightly soluble in water, soluble in DMSO and alcohol, and practically insoluble in chloroform.
Gliclazide is stable at pH 3–7 and should be stored below 25°C in a dry place, protected from light.
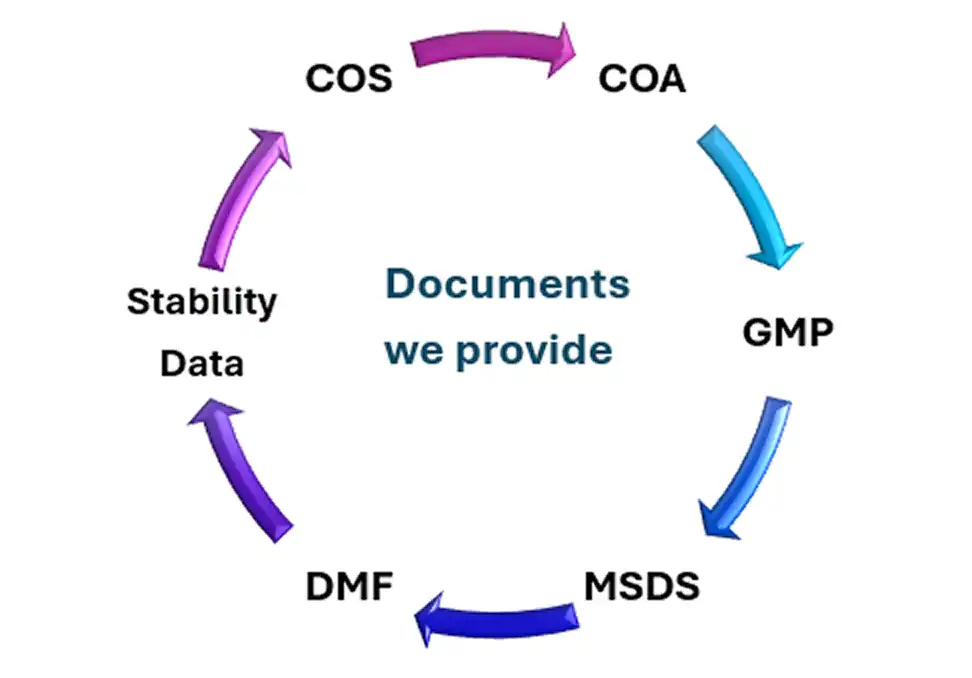
Yes, the API is export-ready, with DMF/CEP support, CoA, MSDS, and stability reports provided for LATAM, MENA, Africa, and Asia markets.
Looking to source Gliclazide or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.