
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
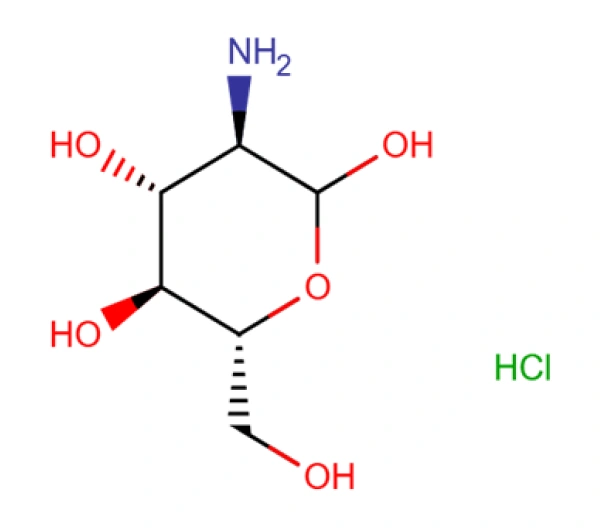
C6H14ClNO5
(3R,4R,5S,6R)-3-amino-6-(hydroxymethyl)oxane-2,4,5-triol hydrochloride
66-84-2
215.63 g/mol
Monosaccharide derivative
Nutritional Supplement
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water; slightly soluble in ethanol; insoluble in organic solvents like chloroform and ether |
| Melting Point | 190–194°C |
| pH | 3.5-5.5 |
Glucosamine HCl is a naturally derived amino sugar and a key building block of cartilage, commonly used as a dietary supplement to support joint health and treat osteoarthritis. It plays a vital role in the synthesis of glycosaminoglycans and proteoglycans, which maintain the structure and function of cartilage. Typically sourced from shellfish or produced synthetically, glucosamine hydrochloride is valued for its ability to reduce joint pain and improve mobility by promoting cartilage repair and reducing inflammation.
Glucosamine hydrochloride works by providing the body with the essential building blocks needed to produce glycosaminoglycans and proteoglycans, which are vital components of cartilage, helping to repair damaged tissue, reduce inflammation, and improve joint function.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Glucosamine HCl is used to support joint health, relieve osteoarthritis symptoms, reduce joint pain and inflammation, and promote cartilage repair.
Glucosamine HCl is typically taken orally in capsule or tablet form, usually once or twice daily with food to improve absorption and reduce stomach upset; follow your healthcare provider’s dosage instructions.
Glucosamine HCl is generally not known to cause low blood sugar, but people with diabetes should monitor their blood sugar closely and consult their healthcare provider before use, as it may potentially affect insulin sensitivity in some cases.
Yes, Salius Pharma Pvt. Ltd. exports Glucosamine Hydrochloride (HCl) API to countries in both Africa and the Middle East. The company is an ISO 9001:2015 certified and Government of India-recognized Export House, actively involved in the manufacturing, marketing, and export of a wide range of pharmaceutical formulations and active pharmaceutical ingredients (APIs) to numerous countries worldwide, including regions in Africa and the Middle East.
Yes, Salius Pharma offers full technical support and regulatory documentation for Glucosamine HCl API, including DMF, CTD, and CEP formats tailored to international standards. The company supports clients with regulatory submissions such as ANDA, ACTD, and country-specific dossiers, ensuring compliance with WHO-GMP, USFDA, and other global authorities. This enables seamless registration and supply of Glucosamine HCl API in African and Middle Eastern markets, aligning with Salius Pharma’s strategic export focus in these regions.
Looking to source Glucosamine HCl or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.