
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
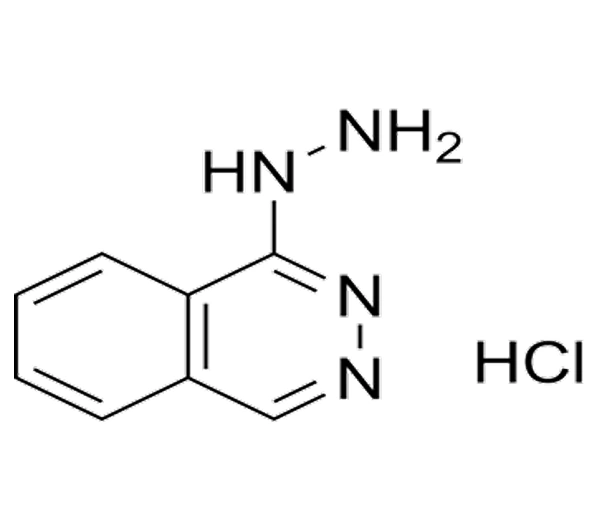
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Hydralazine hydrochloride
C₈H₈N₄ · HCl
~196.64 g/mol
304-20-1
1 hydrazinophthalazine monohydrochloride
Hydrazinophthalazine derivative
Antihypertensive
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Soluble in water; slightly soluble in alcohol; very slightly soluble in ether. |
| Melting Point | ~275 °C (with decomposition) |
| pH | A 2% aqueous solution has pH ~3.5–4.2 |
Hydralazine HCl is a direct-acting vasodilator from the hydrazinophthalazine class, primarily used in the form of hydralazine hydrochloride. It lowers blood pressure by relaxing the smooth muscle of small arteries (arterioles), reducing peripheral vascular resistance, and decreasing the workload on the heart. Hydralazine is absorbed orally, undergoes first-pass metabolism in the liver, and is also available in injectable form for rapid control of severe hypertension or hypertensive emergencies.
Hydralazine HCl works by directly relaxing arteriolar smooth muscle through interference with calcium ion movement and nitric oxide pathways, leading to decreased peripheral resistance and reduced blood pressure. The result is improved cardiac output and reduced afterload without significant effects on venous capacitance vessels.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
The USP, BP, and EP monographs recognize Hydralazine hydrochloride (HCl), not the free base, as the official API form.
Hydralazine HCl is used to treat essential and severe hypertension, hypertensive emergencies, heart failure (as adjunct therapy), and certain pregnancy-related hypertensive conditions.
Direct compression or wet granulation can be used; micronized grades are often preferred to improve uniformity and bioavailability.
Hydralazine HCl is soluble in water, slightly soluble in alcohol, and melts around 275 °C (with decomposition).
Store below 25 °C in a dry place, protected from light; stable under normal conditions, but avoid prolonged exposure to heat and moisture.
Yes, it is export-ready; typical documentation includes CoA, MSDS, impurity profile, and pharmacopeial compliance (USP/BP/EP), with DMF or CEP support where applicable.
Looking to source Hydralazine HCl or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.