
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
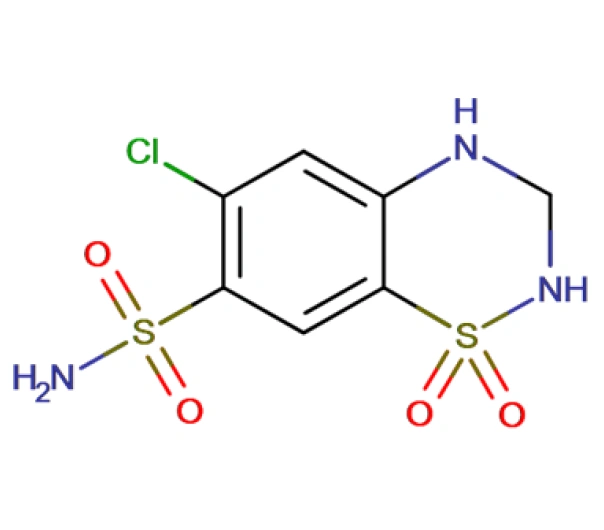
C7H8ClN3O4S2
6-chloro-1,1-dioxo-3,4-dihydro-2H-1lambda6,2,4-benzothiadiazine-7-sulfonamide
58-93-5
297.73 g/mol
benzothiadiazines
Diuretic
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; Freely soluble in sodium hydroxide solution |
| Melting Point | 273–275°C |
| pH | 6.5 |
Hydrochlorothiazide is a thiazide diuretic used to treat high blood pressure and fluid retention caused by conditions like heart failure and kidney disorders. It works by reducing the reabsorption of sodium and chloride in the kidneys, leading to increased urine output and lower blood volume. Taken orally, HCTZ is often combined with other blood pressure medications. Common side effects include low potassium, dizziness, and increased blood sugar. It should be avoided in patients with severe kidney problems or sulfa allergies.
Hydrochlorothiazide (HCTZ) works by inhibiting the sodium-chloride symporter in the distal convoluted tubule of the nephron in the kidneys. This action prevents the reabsorption of sodium (Na⁺) and chloride (Cl⁻) back into the bloodstream, causing them to be excreted in the urine along with water. As a result, it reduces blood volume, which lowers blood pressure. It also increases the excretion of potassium and bicarbonate, which can lead to side effects like hypokalemia (low potassium levels). By promoting diuresis (increased urine production), HCTZ helps reduce swelling (edema) and manage hypertension.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It’s primarily used to treat high blood pressure and reduce fluid retention (edema).
It is generally avoided during pregnancy unless clearly needed, as it can affect blood volume.
Yes, Hydrochlorothiazide can interact with several medications. For example, it may increase the risk of lithium toxicity, reduce the effectiveness of diabetes medications, and its diuretic effect can be diminished by NSAIDs like ibuprofen.
Yes, Salius Pharma exports Hydrochlorothiazide Active Pharmaceutical Ingredients (API) to several countries in Africa and the Middle East. According to their global expansion plan, Salius Pharma is actively targeting markets in Africa, including countries like Nigeria, Kenya, Ghana, and South Africa, as well as the Middle East.
Yes, Salius Pharma Pvt. Ltd. provides comprehensive technical support and documentation for its Hydrochlorothiazide API. As an ISO 9001:2015 certified company and a Government of India-recognized Star Export House, Salius Pharma is committed to delivering high-quality pharmaceutical products and services globally.
Looking to source Hydrochlorothiazide or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.