
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
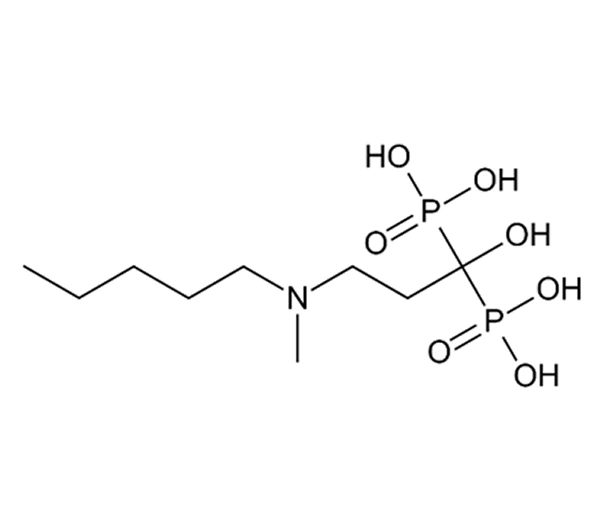
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Ibandronate sodium (monosodium salt, monohydrate)
Approx. C₉H₂₂N O₇P₂·Na·H₂O (salt monohydrate form)
~359.2 g/mol (monohydrate salt form)
138926-19-9 (sodium monohydrate salt)
3-(N methyl-N-pentyl)amino 1 hydroxypropane-1,1-diphosphonic acid, monosodium salt, monohydrate
Nitrogen-containing bisphosphonate derivative
Bone resorption inhibitor
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water; practically insoluble in most organic solvents. |
| Melting Point | 210–215 °C |
| pH | - |
Ibandronate sodium is a potent nitrogen containing bisphosphonate widely used for the prevention and treatment of osteoporosis in post menopausal women. It reduces bone resorption by inhibiting osteoclast activity, thereby helping to maintain or increase bone mineral density (BMD) and lowering the risk of fractures. Beyond osteoporosis, ibandronate has also been used to manage hypercalcemia of malignancy and to prevent skeletal complications in cancers with bone metastases.
Ibandronate binds strongly to bone mineral (hydroxyapatite) at sites of active bone remodeling. Once osteoclasts attempt to resorb bone containing ibandronate, the bisphosphonate is internalized and inhibits the enzyme Farnesyl diphosphate synthase (FDPS), thereby disrupting the mevalonate pathway — essential for prenylation of small GTPase signaling proteins required for osteoclast function. This inhibition reduces osteoclast activity and promotes their apoptosis, leading to decreased bone resorption, reduced bone turnover, and a net gain (or maintenance) of bone mass.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Bone resorption inhibitor; nitrogen-containing bisphosphonate.
Typically supplied as crystalline monohydrate; amorphous forms are rare.
Primarily via renal excretion; part binds to bone for prolonged effect.
Handle in a dry environment; use moisture-protected packing and avoid dust exposure.
Yes; oral monthly dosing is common, IV used for GI intolerance or compliance issues.
Yes, both CoA and MSDS are provided along with pharmacopeial compliance.
Looking to source Ibandronate Sodium or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.