
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP / EP / BP
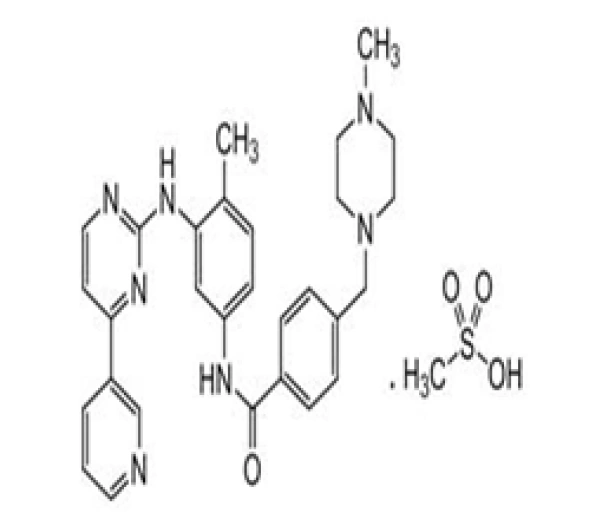
C₂₉H₃₁N₇O · CH₄O₃S
4-[(4-Methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide methanesulfonate
220127-57-1
589.7 g/mol
Tyrosine kinase inhibitor (Anticancer agent)
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in methanol |
| Melting Point | ~214–216 °C (decomposes) |
| pKa | ~8.1 |
Imatinib Mesylate treats Philadelphia chromosome-positive CML in adults and children, acute lymphoblastic leukemia, chronic eosinophilic leukemia, and GIST by inhibiting BCR-ABL tyrosine kinase and other targets.It selectively blocks abnormal kinase activity in cancer cells, reducing proliferation while sparing healthy tissues, with formulations for tablets or capsules. Common adverse effects include nausea, muscle cramps, rash, and fluid retention; it shows a favorable profile over traditional chemotherapies.
Imatinib Mesylate selectively inhibits BCR-ABL tyrosine kinase, the abnormal enzyme responsible for uncontrolled cell growth in CML. It also inhibits KIT and PDGFR tyrosine kinases, making it effective against GIST. By blocking these signaling pathways, Imatinib prevents cancer cell proliferation and induces apoptosis without significantly affecting normal cells.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, shipments include DMF, CEP certificates where applicable, impurity profiles, and stability data to aid filings in regulated markets.
Salius exports worldwide to Asia, Europe, the Middle East, Africa, North America, and South America, complying with local regulations and providing DMF support.
Use requires strict medical supervision with dose adjustments; approved for pediatric CML patients over 2 years and elderly with monitoring for comorbidities.
Store in a cool, dry place away from light and moisture in tightly sealed, tamper-evident containers to maintain stability.
It inhibits BCR-ABL and other kinases in cancer cells, blocking signals for uncontrolled growth and inducing apoptosis while minimizing effects on normal cells.
Imatinib Mesylate treats Philadelphia chromosome-positive chronic myeloid leukemia (CML), gastrointestinal stromal tumors (GIST), and certain leukemias by targeting abnormal tyrosine kinases.
Looking to source Imatinib Mesylate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.