
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
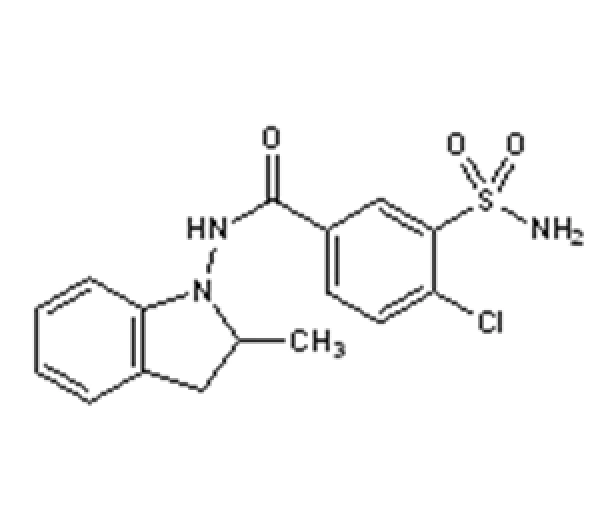
C16H16ClN3O3S
4-Chloro-N-(2-methyl-2,3-dihydro-1H-indol-1-yl)-3-sulfamoylbenzamide
26807-65-8
365.83 g/mol
Sulfonamide derivative / Indoline compound
Thiazide-like diuretic / Antihypertensive agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; freely soluble in methanol, ethanol, and acetone; practically insoluble in ether and chloroform |
| Melting Point | 160 – 162 °C |
| pH (1% aqueous solution) | 5.0 – 7.0 |
Indapamide is a thiazide-like diuretic (water pill) used primarily to treat high blood pressure (hypertension) and fluid retention (edema) caused by congestive heart failure. Indapamide is known for its excellent bioavailability, long duration of action, and minimal metabolic side effects, making it a preferred choice in modern cardiovascular therapy.
Indapamide acts primarily as a thiazide-like diuretic that reduces blood pressure through a dual mechanism: (1) Renal Action: It inhibits sodium and chloride reabsorption in the distal convoluted tubule of the nephron, leading to increased excretion of sodium, chloride, and water. This mild diuretic effect helps decrease plasma volume and, consequently, lowers cardiac output and blood pressure. (2) Vascular Action: Indapamide also exhibits direct vasodilatory properties by reducing calcium influx into vascular smooth muscle cells, resulting in decreased peripheral vascular resistance. These combined effects lead to a sustained antihypertensive response with minimal metabolic disturbances compared to conventional thiazide diuretics.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Our Indapamide API complies with BP/USP standards and is supported by complete regulatory documentation, including COA, DMF (where applicable), and CTD dossiers. All batches are manufactured under cGMP and ISO-certified facilities.
We export globally with a strong focus on LATAM, MENA, CIS, and Southeast Asia regions. Our products meet the import and registration requirements of multiple regulatory authorities worldwide.
Indapamide is packed in HDPE drums or fiber containers, lined with double polyethylene bags to ensure protection against moisture and contamination. Shipments are handled under standard export conditions — via air or sea freight, depending on client preference and urgency.
Yes. We provide comprehensive regulatory support, including DMF filing assistance, technical data, and product dossiers in CTD format to facilitate product registration in your country.
Our standard MOQ is 25 kg, but we accommodate smaller trial orders for regulatory submissions or formulation development upon request.
Looking to source Indapamide or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.