
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
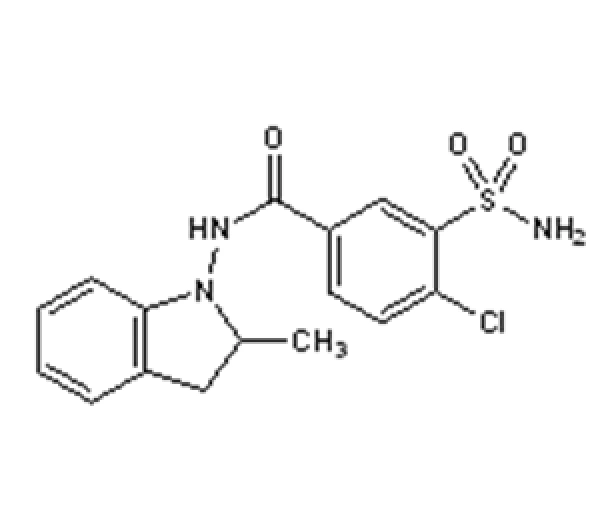
C19H16ClNO4
1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic acid
53-86-1
357.79 g/mol
Indole acetic acid derivative / Nonsteroidal anti-inflammatory compound (NSAID)
Nonsteroidal anti-inflammatory drug (NSAID) / Analgesic / Antipyretic
| Appearance | White to pale yellow crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; freely soluble in ethanol, methanol, dimethylformamide, and slightly soluble in chloroform and ether |
| Melting Point | 160 – 162 °C |
| pH (1% aqueous solution) | 4.0 – 5.0 |
Indomethacin is a potent nonsteroidal anti-inflammatory drug (NSAID) used to relieve moderate to severe pain, swelling, and stiffness from inflammation. It works by inhibiting the synthesis of prostaglandins, chemical messengers in the body that cause pain, fever, and inflammation.
Indomethacin acts by inhibiting cyclooxygenase (COX-1 and COX-2) enzymes, which are responsible for the biosynthesis of prostaglandins—key mediators of pain, inflammation, and fever. By reducing prostaglandin formation, Indomethacin produces anti-inflammatory, analgesic, and antipyretic effects. It also decreases vasodilation, edema, and leukocyte migration at sites of inflammation, making it a potent nonsteroidal anti-inflammatory agent (NSAID).
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Our Indomethacin API complies with BP/USP standards and is supported by comprehensive regulatory documentation, including COA, MSDS, CTD Dossier, and DMF (upon request). All production follows cGMP and ISO-certified manufacturing protocols.
We supply Indomethacin globally, with a strong presence in Latin America, MENA, CIS, and Southeast Asia. Our documentation and quality standards are aligned with the requirements of major international health authorities.
Each export batch is shipped with the following documents: Certificate of Analysis (COA), Material Safety Data Sheet (MSDS), GMP / ISO Certificates, Packing List, Invoice & Batch Details. Additional regulatory support documents such as DMF Access Letters or CTD Modules are available on request.
Indomethacin is packed in HDPE or fiber drums with double-layer polyethylene liners, ensuring moisture protection and product integrity. Packaging sizes are customizable (1 kg to 25 kg) based on client needs.
All consignments are shipped via air or sea freight under standard pharmaceutical export conditions. We partner with certified freight forwarders experienced in handling regulated APIs, ensuring safe and timely delivery.
Every shipment is sealed, labeled, and moisture-protected using industry-standard packaging materials. We ensure compliance with Good Distribution Practices (GDP) and maintain complete traceability from production to delivery.
Looking to source Indomethacin or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.