
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
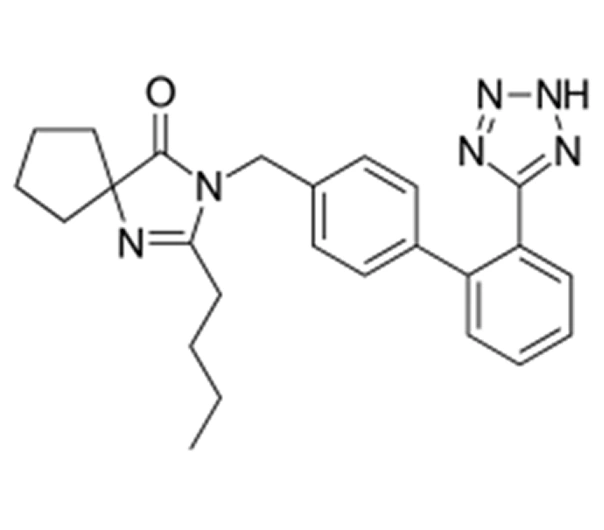
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Irbesartan
C₂₅H₂₈N₆O
~428.5 g/mol
138402-11-6
2-butyl-3-{[2′-(1H tetrazol-5 yl)biphenyl-4-yl]methyl}-1,3 diazaspiro[4.4]non-1 en-4-one
Non-peptide Angiotensin II AT₁ receptor antagonist
Antihypertensive
| Appearance | White to off white, hygroscopic, crystalline powder |
|---|---|
| Solubility | Slightly soluble in alcohol/methylene chloride; practically insoluble in water. |
| Melting Point | - |
| pH | - |
Irbesartan is a widely used, orally active, non-peptide angiotensin II type 1 (AT₁) receptor antagonist (ARB). It lowers blood pressure and provides renal-protective benefits (especially in patients with type 2 diabetes and proteinuria) by blocking angiotensin II–mediated vasoconstriction and aldosterone secretion, leading to vasodilation, reduced sodium and water retention, and decreased peripheral vascular resistance.
Irbesartan selectively binds and blocks the AT₁ receptor for angiotensin II, preventing angiotensin II’s effects — vasoconstriction, aldosterone release, sodium/water retention — thereby relaxing vascular smooth muscle and lowering blood pressure. It does not inhibit ACE or affect bradykinin pathways.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Food has minimal effect on absorption; bioavailability remains largely unchanged. It can be taken with or without food without impacting therapeutic effect. No dose adjustment is required based on meals.
Irbesartan is slightly hygroscopic, especially under high-humidity conditions. It should be stored in tight, moisture-protected containers. Controlled humidity (<60% RH) is recommended for handling.
Yes—US-DMF, EU-DMF, and other CTD-format dossiers are commonly available from compliant manufacturers. DMFs help support filings in the US, EU, Japan, and other regulated regions. Availability may vary—buyers typically receive DMF status upon request.
Yes, QC testing usually follows USP and/or Ph. Eur monograph methods. Typical tests include assay, impurities, ID, water content, and residual solvents. Validated in-house methods may also be supplied for non-compendial parameters.
Yes—retest periods are established per ICH Q1A (stability) and intermediate/accelerated conditions. Common retest periods range from 24–48 months, depending on stability data. Stability summaries are included in the CoA/CTD package.
Looking to source Irbesartan or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.