
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP
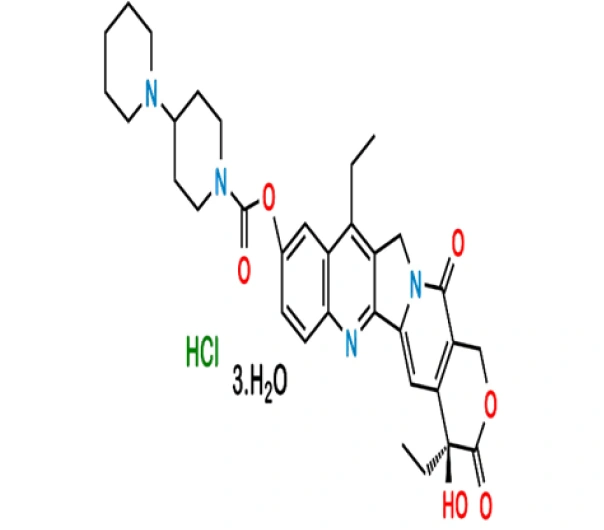
C₃₃H₃₈ClN₄O₆·3H₂O
(4S)-4,11-diethyl-4-hydroxy-9-[(4-piperidin-1-ylpiperidin-1-yl)carbonyl]-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione hydrochloride trihydrate
136572-09-3
677.20 g/mol
Semisynthetic camptothecin derivative
Anti-Cancer
| Appearance | Pale yellow to greenish crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in methanol |
| Melting Point | 256.5°C |
| pH | - |
Irinotecan HCl Trihydrate is a semisynthetic camptothecin derivative used as a chemotherapy agent primarily for treating metastatic colorectal cancer and other solid tumors. It works by inhibiting topoisomerase I, an enzyme essential for DNA replication, leading to DNA damage and cancer cell death.

Irinotecan Hydrochloride Trihydrate works by inhibiting the enzyme topoisomerase I, which is crucial for relieving DNA supercoiling during replication. By stabilizing the complex between topoisomerase I and DNA, irinotecan prevents the re-ligation (repair) of single-strand DNA breaks. This leads to the accumulation of DNA damage, ultimately causing cancer cell death through apoptosis. This mechanism makes irinotecan effective in stopping the growth of rapidly dividing cancer cells.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It is used primarily to treat metastatic colorectal cancer and small-cell lung cancer.
Irinotecan is usually administered intravenously under medical supervision.
Early-onset diarrhea may be treated with atropine, while late-onset diarrhea requires prompt use of loperamide and hydration.
Yes, Salius Pharma Pvt. Ltd. exports Irinotecan Hydrochloride Trihydrate API to various countries, including those in Africa and the Middle East. As an ISO 9001:2015 certified company recognized as a Government of India Star Export House, Salius Pharma has a strong presence in international markets.
Yes, Salius Pharma offers robust technical support and documentation for their Irinotecan Hydrochloride Trihydrate API. As a recognized ISO 9001:2015–certified and Government of India Star Export House, they maintain a dedicated Regulatory Affairs team skilled in providing critical technical documents—including US/EU Drug Master Files (DMFs), Certificates of Suitability (CEPs), and CTD/eCTD dossiers—for seamless registration and market access across regulated and non-regulated territories.
Looking to source Irinotecan HCl Trihydrate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.