
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Parenteral Active Pharmaceutical Ingredient (API)
USP / EP (as applicable)
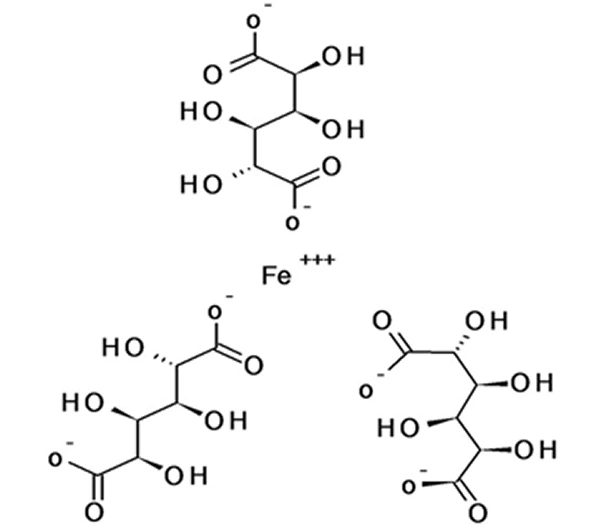
Iron(III)-hydroxide sucrose complex
Approx. C₁₂H₂₉Fe₅O₂₃ (polymeric complex; varies by ligand structure)
34,000–60,000 Da (average polymeric complex)
8047-67-4
Iron(III)-oxo-hydroxide sucrose complex (polymeric; no single fixed IUPAC name)
Parenteral iron–carbohydrate complex
Intravenous anti-anemic agent (IV iron for iron-deficiency anemia)
| Appearance | Dark brown |
|---|---|
| Solubility | Fully soluble in water (as injection) |
| Melting Point | - |
| pH | - |
Iron Sucrose is a parenteral iron complex composed of polynuclear iron (III)-hydroxide bound to sucrose, used for rapid correction of iron deficiency when oral iron is ineffective or not tolerated. It offers excellent bioavailability, predictable dosing, and a strong safety profile, making it one of the most widely used IV iron therapies globally.
Provides bioavailable iron (III) directly to the reticuloendothelial system. Iron is gradually released and incorporated into hemoglobin and ferritin. Bypasses gastrointestinal absorption, ensuring rapid repletion of iron stores.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, most manufacturers perform stability studies as per ICH Zone IVb (30°C / 75% RH) to support exports to tropical markets. This ensures the product remains stable in high-temperature, high-humidity regions.
Iron Sucrose typically conforms to USP and EP monographs. Manufacturers usually offer pharmacopeial-compliant grades depending on the regulatory requirements of the destination market.
Sucrose degradation can occur under extreme heat or acidic conditions, but when stored properly (cool, protected from light), degradation is minimal. Stability is maintained within standard storage conditions.
Iron Sucrose is commonly packed in epoxy-lined or HDPE drums with sterile, light-protected inner liners. For small-volume or sensitive batches, amber glass containers may also be used to prevent light exposure.
Looking to source Iron Sucrose or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.