
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
Active Pharmaceutical Ingredient (API)
IP / JP / In-house GMP Grade
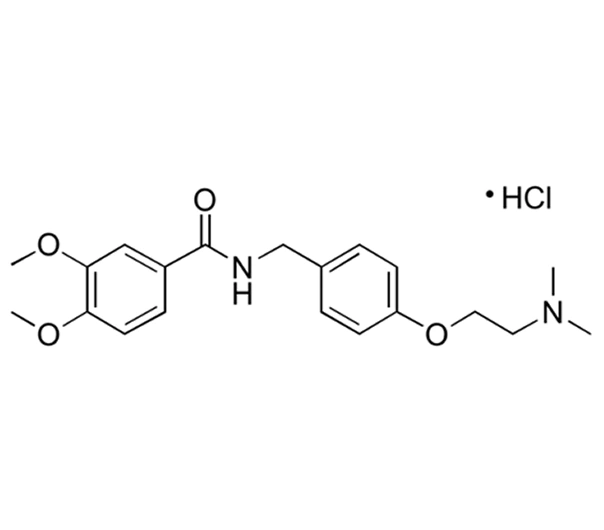
Itopride Hydrochloride
C₂₀H₂₆N₂O₄·HCl
~394.89 g/mol (HCl salt)
122898-67-3
N-[4-[2-(dimethylamino)ethoxy]benzyl]-3,4-dimethoxybenzamide hydrochloride
Benzamide derivative
Prokinetic agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water, methanol; slightly soluble in ethanol. |
| Melting Point | - |
| pH | - |
Itopride Hydrochloride is a gastroprokinetic agent widely used for managing functional dyspepsia and impaired gastric motility. It works through dual action—dopamine D₂ receptor antagonism and acetylcholinesterase inhibition—leading to enhanced gastric emptying and improved gastrointestinal transit. Due to its strong efficacy and minimal CNS penetration, Itopride HCl is preferred in various global markets for treating motility disorders.
Dopamine D₂ receptor antagonism increases acetylcholine release in the GI tract. Acetylcholinesterase inhibition prolongs acetylcholine action, enhancing motility. Improves gastric emptying and reduces dyspepsia symptoms without significant CNS side effects.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Nitrogen flushing is not mandatory because Itopride HCl is not highly oxidatively sensitive. However, some manufacturers use nitrogen flushing as an added precaution for long-term storage in humid or tropical export markets.
Yes, Itopride HCl is generally suitable for both wet granulation and direct compression. Formulators often prefer wet granulation for better flow and compressibility, but PSD-controlled grades can support direct compression.
Yes, GTI risk assessment is usually conducted as per ICH M7 guidelines. Manufacturers monitor potential GTIs in the synthetic route and ensure they remain below acceptable limits.
Most suppliers provide non-sterile API, but low-bioburden grades can be offered upon request. Sterile API is uncommon but may be available through specialized facilities with validated sterilization steps.
Looking to source Itopride Hydrochloride or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.