
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
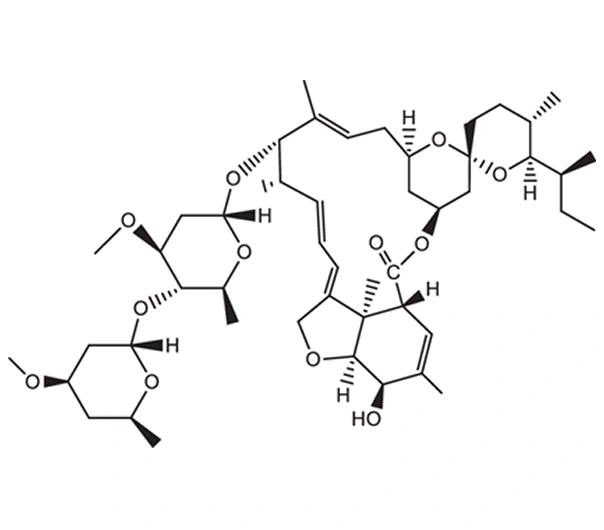
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Ivermectin
C₄₈H₇₄O₁₄
~875.1 g/mol
70288-86-7
22,23-Dihydroavermectin B1a + B1b (mixture of 80:20)
Macrocyclic lactone
Anthelmintic; Anti-parasitic agent
| Appearance | White to yellowish crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; soluble in ethanol, methanol, acetone, chloroform |
| Melting Point | 155–157°C (B1a), 145–150°C (B1b) |
| pH | - |
Ivermectin is a broad-spectrum antiparasitic agent widely used for the treatment of a variety of internal and external parasitic infections in humans and animals. It acts by binding selectively to parasite-specific glutamate-gated chloride channels, leading to paralysis and death of the parasite. Its high efficacy, safety profile, and oral bioavailability make it a preferred choice in both human and veterinary medicine.
Ivermectin works by binding selectively to parasite-specific glutamate-gated chloride channels, which increases chloride ion permeability and leads to hyperpolarization of nerve and muscle cells. This disruption causes paralysis and eventual death of the parasite, while having minimal effect on mammalian chloride channels, thereby providing a high safety margin for human and veterinary use.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Commercial Ivermectin API is typically supplied as a mixture of B1a and B1b, with an approximate ratio of 80:20. This ratio is controlled to ensure consistent potency and pharmacological activity.
Yes, stability studies for Ivermectin are conducted following ICH Q1A(R2) guidelines. Both long-term and accelerated conditions are evaluated to ensure shelf-life and quality in various climates.
Yes, microbial limits are monitored, especially for low-bioburden or parenteral applications. Standard microbiological testing ensures safety during handling and formulation.
Yes, every batch is accompanied by a Certificate of Analysis (CoA), Material Safety Data Sheet (MSDS), and TSE/BSE declaration for regulatory compliance and safe handling.
Looking to source Ivermectin or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.