
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
EP/USP
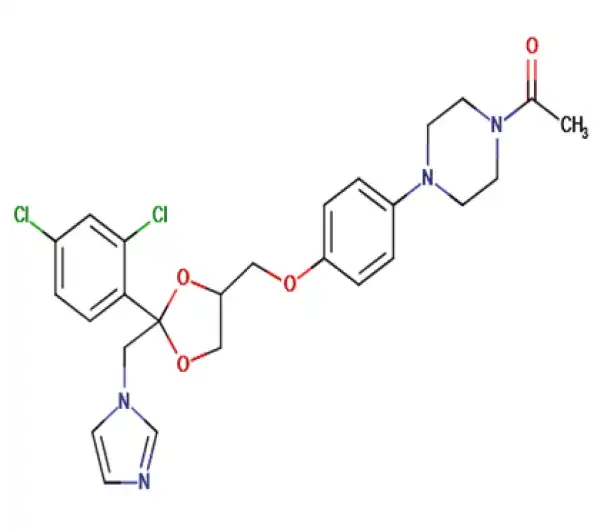
C26H28Cl2N4O4
1-[4-(4-{[2-(2,4-dichlorophenyl)-2-[(1H-imidazol-1-yl)methyl]-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin-1-yl]ethan-1-one
65277-42-1
531.43 g/mol
Imidazole
Anti-Fungal
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; soluble in acetone, methanol, and dichloromethane |
| Melting Point | 146–150 °C |
| pH | - |
Ketoconazole is a broad-spectrum antifungal agent belonging to the imidazole class. It is used to treat various fungal infections, including dermatophytosis, candidiasis, and seborrheic dermatitis. Ketoconazole works by inhibiting the synthesis of ergosterol, an essential component of fungal cell membranes, leading to increased membrane permeability and fungal cell death. Available in topical and oral formulations, it is commonly prescribed for both superficial and systemic fungal infections. However, due to potential liver toxicity, oral use is limited and carefully monitored.
Ketoconazole works by inhibiting the fungal enzyme lanosterol 14α-demethylase, a type of cytochrome P450 enzyme. This inhibition blocks the conversion of lanosterol to ergosterol, an essential sterol component of the fungal cell membrane. Without ergosterol, the fungal cell membrane becomes unstable and permeable, disrupting cell function and leading to fungal cell death. This mechanism makes ketoconazole effective against a wide range of fungal infections.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |

When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Ketoconazole treats fungal infections like athlete’s foot, ringworm, yeast infections, and seborrheic dermatitis.
It inhibits fungal ergosterol synthesis by blocking lanosterol 14α-demethylase, disrupting the fungal cell membrane.
Oral ketoconazole is not recommended during pregnancy; topical forms should be used with caution.
Yes, Salius Pharma Pvt. Ltd. exports Ketoconazole API to various countries, including those in Africa and the Middle East. As an ISO 9001:2015 certified company recognized as a Government of India Star Export House, Salius Pharma has a strong presence in international markets. They have a history of exporting pharmaceutical products to destinations such as the United Arab Emirates, Nigeria, and other countries in Africa and the Middle East.
Yes, Salius Pharma Pvt. Ltd. provides comprehensive technical support and documentation for Ketoconazole API, including regulatory dossiers in CTD/eCTD formats, Drug Master Files (DMFs) filed with US FDA and EU authorities, Certificates of Suitability (CEPs) compliant with European Pharmacopoeia standards, and detailed Certificates of Analysis (COAs). Their manufacturing facilities are certified by WHO-GMP, USFDA, TGA Australia, and UK MHRA, ensuring high-quality standards.
Looking to source Ketoconazole or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.