
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP/EP
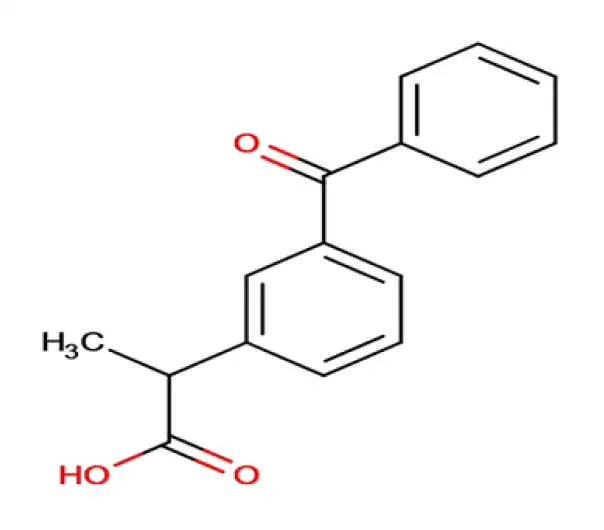
C16H14O3
2-(3-benzoylphenyl)propanoic acid
22071-15-4
254.28 g/mol
arylpropionic acid derivatives
NSAIDs
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Slightly soluble in water; soluble in ethanol, methanol, and acetone |
| Melting Point | 94–97°C |
| pH | 4.45 |
Ketoprofen is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce inflammation, pain, and fever. It works by inhibiting cyclooxygenase enzymes (COX-1 and COX-2), which decreases the production of prostaglandins responsible for inflammation and pain. Ketoprofen is commonly prescribed for conditions like arthritis, muscle pain, and postoperative pain. It is available in oral, topical, and injectable forms and is valued for its effectiveness in managing both acute and chronic inflammatory conditions.
Ketoprofen works by inhibiting the enzymes cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), which are responsible for converting arachidonic acid into prostaglandins. Prostaglandins are chemicals that mediate inflammation, pain, and fever. By blocking COX enzymes, ketoprofen reduces the production of prostaglandins, leading to decreased inflammation, pain relief, and reduced fever.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
It’s used to relieve pain, inflammation, and fever in conditions like arthritis, muscle pain, and menstrual cramps.
It inhibits COX enzymes, reducing prostaglandin production which lowers pain and inflammation.
It can interact with blood thinners, other NSAIDs, and certain blood pressure medications—consult your doctor.
Yes, Salius Pharma Pvt. Ltd. exports Ketoprofen API to various countries, including those in Africa and the Middle East. As an ISO 9001:2015 certified company recognized as a Government of India Star Export House, Salius Pharma has a strong presence in international markets. They have a history of exporting pharmaceutical products to destinations such as the United Arab Emirates, Nigeria, and other countries in Africa and the Middle East.
Yes, Salius Pharma Pvt. Ltd. provides comprehensive technical support and documentation for Ketoprofen API, including regulatory dossiers (CTD/eCTD), Drug Master Files (DMFs), Certificates of Suitability (CEPs), and detailed Certificates of Analysis (COAs), ensuring compliance with global standards through their WHO-GMP, USFDA, TGA Australia, and UK MHRA-certified manufacturing facilities.
Looking to source Ketoprofen or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.