
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
BP/USP
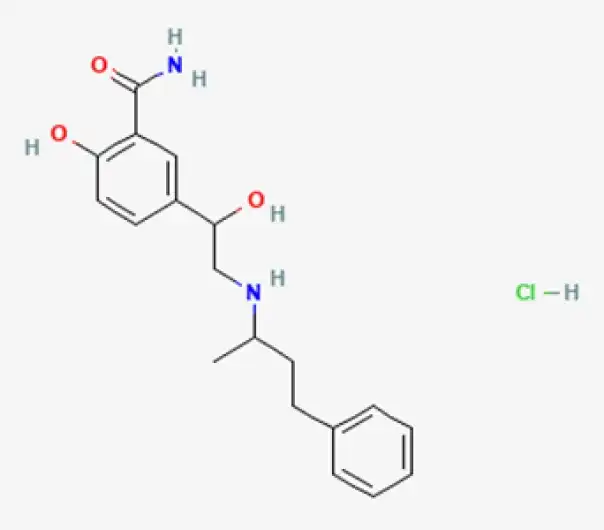
C19H24N2O3·HCl
2-hydroxy-5-[1-hydroxy-2-(1-methyl-3-phenylpropylamino)ethyl]benzamide hydrochloride
32780-64-6
364.86 g/mol
Adrenergic receptor blocker
Antihypertensive agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water and methanol; slightly soluble in ethanol; practically insoluble in chloroform and ether |
| Melting Point | 178 – 182 °C (with decomposition) |
| pH (1% aqueous solution) | 4.0 – 5.0 |
Labetalol Hydrochloride is a combined α- and β-adrenergic receptor blocker used primarily in the management of hypertension. It offers a unique dual mechanism that provides effective blood pressure control without significant reflex tachycardia. Labetalol’s balanced blocking action results in reduced peripheral vascular resistance (via α-blockade) while maintaining cardiac output and heart rate control (via β-blockade).
Labetalol competitively blocks β1-, β2-, and α1-adrenergic receptors:
• β-blockade reduces heart rate, cardiac output, and renin release.
• α-blockade causes vasodilation, lowering peripheral vascular resistance.
The combination results in balanced antihypertensive activity without significant changes in resting heart rate or cardiac output.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Our Labetalol HCl is produced in GMP- and ISO-certified facilities with rigorous quality control and validated processes. Each batch meets BP/USP standards, ensuring consistent purity, potency, and global compliance.
Yes. We provide region-specific regulatory support, including CTD/ACTD formats, DMF access letters, and stability data tailored to local authority requirements, simplifying product registration in your market.
We collaborate with certified pharmaceutical freight partners to ensure temperature-controlled, moisture-protected, and traceable shipments. Clients can choose between air, sea, or courier modes for flexible delivery timelines.
Our facilities undergo periodic internal and third-party audits for GMP, ISO 9001:2015, and WHO compliance. Certificates and audit summaries can be shared with partners for their internal vendor qualification.
Yes. We offer annual or multi-year supply contracts, with guaranteed pricing and volume commitments. Our supply chain team ensures inventory planning and timely replenishment to support uninterrupted production for our clients.
Looking to source Labetalol HCl or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.