
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
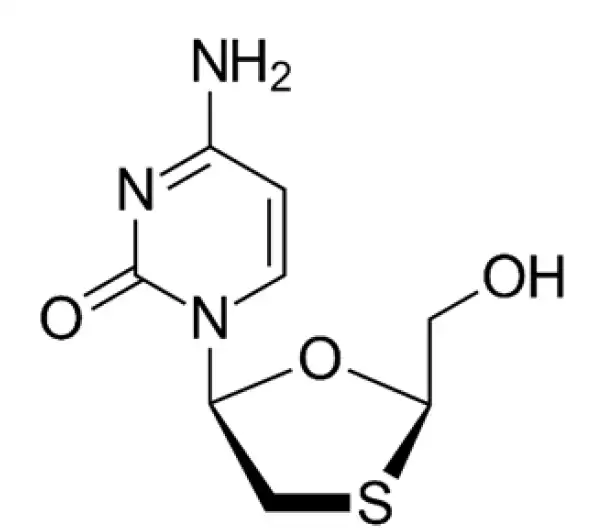
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Lamivudine
C₈H₁₁N₃O₃S
~229.26 g/mol
134678-17-4
(2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-1H-pyrimidin-2-one
Nucleoside reverse transcriptase inhibitor (NRTI)
Antiretroviral; Anti-HIV agent
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Freely soluble in water; slightly soluble in ethanol; insoluble in chloroform |
| Melting Point | 176–178°C |
| pH | 4.5–5.5 |
Lamivudine is an antiretroviral nucleoside analogue used primarily in the treatment of HIV-1 infection and chronic hepatitis B. It acts as a reverse transcriptase inhibitor, blocking viral replication by causing chain termination during DNA synthesis. Lamivudine is often combined with other antiretroviral agents for highly active antiretroviral therapy (HAART) due to its potent efficacy, oral bioavailability, and favorable safety profile.
Lamivudine is phosphorylated intracellularly to its active triphosphate form. It competes with natural deoxycytidine triphosphate for incorporation into viral DNA and causes chain termination, inhibiting viral reverse transcriptase. It also exhibits selective inhibition of viral polymerases with minimal effect on human DNA polymerases.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Lamivudine is generally compatible with standard excipients such as lactose, MCC, and starch. No significant incompatibilities have been reported under typical formulation and storage conditions.
Lamivudine is supplied as the free base. No salt forms are typically used in commercial API shipments.
Yes, all residual solvents are monitored and controlled as per ICH Q3C guidelines. Validated analytical methods ensure levels remain within pharmacopeial limits.
The typical retest period is 36 months when stored below 25°C in a dry, light-protected environment—testing ensures continued compliance with quality and stability specifications.
Under recommended storage conditions, Lamivudine is stable and non-caking. Use of moisture-protective packaging prevents agglomeration in humid climates.
Looking to source Lamivudine or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.