
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
API
USP / EP / BP
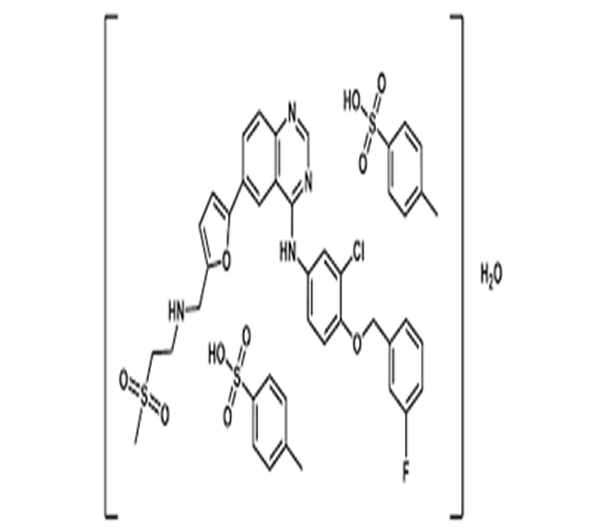
C₂₉H₂₆ClFN₄O₄S · 2C₇H₈O₃S · H₂O
N-(3-chloro-4-((3-fluorobenzyl)oxy)phenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine ditosylate monohydrate
231277-92-2
925.51 g/mol
Tyrosine kinase inhibitor (Anticancer agent)
| Appearance | Yellow to pale-yellow crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; soluble in DMSO and methanol |
| Melting Point | ~137–140 °C (decomposes) |
| pKa | ~7.2 |
Lapatinib Ditosylate Monohydrate is a dual tyrosine kinase inhibitor used primarily in the treatment of HER2-positive breast cancer. It inhibits both epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor-2 (HER2), which play a critical role in tumor cell proliferation. Supplied as a yellow to pale-yellow crystalline powder, it complies with USP, EP, and IP standards and is widely used as an API in solid oral dosage forms such as tablets.
Lapatinib selectively inhibits the intracellular tyrosine kinase domains of EGFR and HER2 receptors. By blocking phosphorylation and downstream signaling pathways, it prevents tumor cell growth and induces apoptosis. This dual inhibition makes it effective in HER2-overexpressing cancers, especially in cases resistant to other therapies.

| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Lapatinib Ditosylate Monohydrate treats HER2-positive metastatic breast cancer, often combined with capecitabine, by targeting EGFR and HER2 tyrosine kinases in tumor cells.
It reversibly inhibits HER1/EGFR and HER2 signaling pathways, blocking cell proliferation and survival signals in HER2-overexpressing cancers.
Batches achieve >99% HPLC purity with full impurity profiling, complying with USP/EP monographs and providing stability data.
Use protective equipment due to potential skin/eye irritation; avoid inhalation of dust and ensure proper ventilation in GMP facilities.
The monohydrate form exhibits high stability with a melting point around 246°C, suitable for long-term storage in sealed packaging under cool, dry conditions.
Looking to source Lapatinib Ditosylate Monohydrate or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.