
API's
Active Pharmaceutical Ingredients

Active Pharmaceutical Ingredients
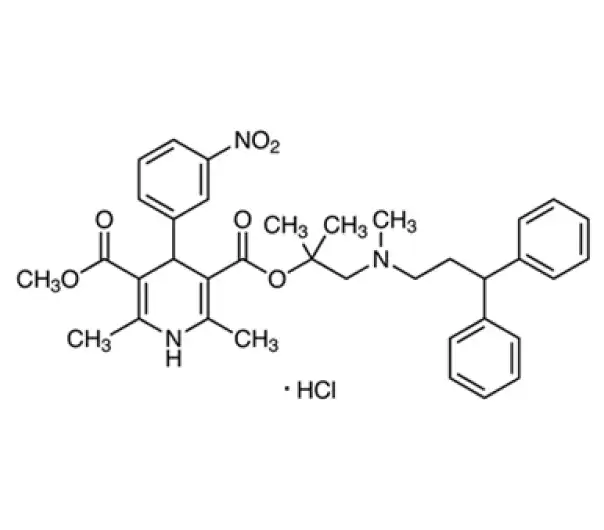
Active Pharmaceutical Ingredient (API)
USP / BP / EP (as applicable)
Lercanidipine Hydrochloride
C₂₁H₂₄ClN₃O₆·HCl
~491.9 g/mol
82832-14-2
(R)-(+)-2-[(3,3-diphenylpropyl)methylamino]ethyl methyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate hydrochloride
Dihydropyridine calcium channel blocker
Antihypertensive
| Appearance | White to off-white crystalline powder |
|---|---|
| Solubility | Practically insoluble in water; soluble in ethanol, methanol, and chloroform |
| Melting Point | 180–182°C |
| pH | - |
Lercanidipine Hydrochloride is a third-generation dihydropyridine calcium channel blocker used for the treatment of hypertension and angina. It selectively inhibits L-type calcium channels in vascular smooth muscle, causing vasodilation, reduced peripheral resistance, and decreased blood pressure. It has high lipophilicity, allowing for gradual onset and prolonged antihypertensive effect with minimal reflex tachycardia.
Lercanidipine works by inhibiting L-type calcium channels in vascular smooth muscle, which causes arterial vasodilation and reduces peripheral resistance. This action lowers both systolic and diastolic blood pressure without significantly affecting heart rate. Its high lipophilicity allows for prolonged tissue retention, resulting in a sustained antihypertensive effect.
| Attribute | Why Salius Pharma Is a Good Choice |
|---|---|
| Quality Certification | WHO GMP, ISO 9001:2015, FDA audited facilities |
| Affordability & Generic Access | Cost competitive generics for global supply |
| Export Experience | Proven track record of supplying globally |
When you order from Salius Pharma, you receive more than just a product — you receive a complete, professionally prepared package that meets international regulatory and quality expectations.
We ensure prompt and reliable shipping worldwide. Delivery times vary depending on destination, order volume, and regulatory requirements.
7 to 21 business days from dispatch, depending on your location.
Yes, all regulatory documentation including Certificate of Analysis (CoA), Material Safety Data Sheet (MSDS), and DMF/CEP support is provided with every batch for export and regulatory compliance.
Yes, the API is manufactured in fully cGMP-compliant facilities with validated processes and quality control methods.
Yes, customized grades and particle size distributions are available to suit specific tablet, capsule, or formulation requirements.
Yes, nitrogen-flushed packing, HDPE drums, or multi-layer liners are available to ensure long-term stability in tropical or high-humidity climates.
Yes, long-term and accelerated stability studies are conducted following ICH Q1A(R2) guidelines, including reports for tropical zones (Zone IVb).
Looking to source Lercanidipine HCl or other high-quality pharmaceutical products?
We’re here to help.
Whether you need Active Pharmaceutical Ingredients (APIs), finished formulations, or regulatory support,
our team is ready to provide dependable, compliant, and cost-effective solutions tailored to your market needs.
The contents of this page and any attachments are intended solely for the designated recipient(s) and may contain confidential and/or privileged information protected by law.
Any patented products are excluded from our offerings in regions where such patents are currently in force.